1. Introduction
Hepatocellular carcinoma (HCC) is a prevalent hepatic disease worldwide, being the cause of 600 thousand deaths per year [1]. It is the sixth most common neoplasm in the world, presenting yearly incidence of one million cases, where HCC is the third related cause of cancer death [2,3].
The pathogenesis of HCC is multifactorial, highly associated to chronic viral hepatitis, alcohol consumption, hepatics toxins, as well as genetic modification, such as hemochromatosis or lack of alpha 1-antitrypsin [4]. Hepatitis B genotype C, high viral load (HBV DNA level > 100,000 copies/mL) and hepatitis C genotype 1b patients with cirrhosis and concurrent esophageal varices have been described as predictors of higher risk of HCC development [5-9]. Liver cirrhosis, male, alcoholism and previous blood transfusion are also important risk factors, and the identification of the metabolic syndrome related to obesity and diabetes was an important cause discovered recently [10-12].
In Brazil, liver cirrhosis is the main risk factor for HCC development and hepatitis C infection is the most common etiology of liver cirrhosis [13]. More than 90% of HCC patient’s present associated liver cirrhosis, leading to clinical symptoms at the end-stage disease, including ascites, hemorrhage caused by varices, peripheral edema, hepatic encephalopathy, and also symptoms related to tumor progression [14].
Treatment of patients with early stage disease (30% from the cases) presents more effective alternatives, such as surgical resection, liver transplantation or percutaneous ablations, which may increase survival and may guarantee a complete response. However, for all other stages the treatments are only palliative in order to relief symptoms, in order to provide to patients a final less painful life [15,16].
The surgical resection and hepatic transplantation are the most curative potential therapy; nevertheless, just a small portion of patients presents themselves in a way of getting some benefit from this treatment. The other ones may get the palliative treatment in order to guarantee an increased survival and an improvement of quality of life [17].
Whatever the state of the patient entering the terminal phase of the illness (Figure 1), it is essential that he or she receives adequate medical, spiritual, and psychological support [18]. The psychological care is essential to patients diagnosed with cancer who also feel getting his death sentence. The terminally ill patient is not a cadaver, but someone who is living your life intensely and needs to understand death and dying process [19].
The last days, hours, and minutes of the life will most probably remain forever in the family’s minds. Therefore, the end of life is of a paramount importance, the medical

Figure 1. Importance of palliative care in all of disease stage for the patient with cancer. Modified from Masera et al. [18].
and psychosocial team is responsible for the correct, appropriate and sensitive management of this moment. It is the responsibility of the health care team to provide adequate pain control, along with control of other noxious symptoms. No less important is the avoidance of unnecessary suffering, both of a dying patient and the surrounding relatives, when death is imminent and all therapeutic modalities have been exhausted [18,20]. However, few studies had as target the end-stage evaluation of HCC diagnosed patients.
Actually, most of the people are not a candidate for a liver transplantation [21]. In spite of an increasing number of people dying on end-stage hepatic diseases, few is reported on the challenge and practical implication on the end-stage patients, as well as the difficulties faced by the patients, family and service providers [22].
2. Palliative Care
The way of informing the patient related to a serious disease diagnosis varies from country to country, due to cultural and religious aspects. In Brazil, a study along with a population from a public university hospital proved a wish of more and deep information on treatment [23, 24]. Many HCC patients were discharged from the hospice even though their status was terminal because of their strong cultural belief that they should die in their own homes [14].
According to World Health Organization, palliative care is an approach that improves the quality of life of patients and their families facing the problem associated with life-threatening illness, through the prevention and relief of suffering by means of early identification and impeccable assessment and treatment of pain and other problems, physical, psychosocial and spiritual [25].
The palliative treatment is an option for, in average, half of the hepatocellular carcinoma diagnosed patients. The goal of this treatment is to guarantee an improvement in survival, but on the other hand, also to guarantee life quality [16].
For patients with hepatic decompensation and with no indication of being through any existing treatments, because of vascular invasion or extrahepatic metastasis, the palliative care is the standard treatment, including radiotherapy in order to eliminate the pain. For those cases where there is an extrahepatic lesion and the liver is compensated, a systemic chemotherapy is sometimes applied, but with no evidence of any benefits [26].
Firstly, it is necessary that patient understands the process of death and dying in order to deal with any phase of its new treatment (palliative care). When his death is understood, it becomes easier to accept. Therefore, it is necessary to realize the “moment” that patient will be prepared to receive the most appropriate and humane care [19].
The death of a large proportion of the population in end-stage HCC emphasises the need for all health care workers to be adequately trained in palliative care in addition to training in complex needs assessment. It should be developed and implemented models for collaboration between primary and secondary care practitioners with palliative care specialists [27].
Palliative Treatments Approach for HCC
The natural history and current therapeutic strategies for patients within different Barcelona Clinic Liver Cancer (BCLC) categories are shown in Figure 2 [29].
The treatment applied is divided in two groups, treatment with curative intention as resection surgery, liver transplantation, local ablation via percutaneous ethanol injection or radiofrequency ablation, and palliative treatments such as transarterial chemoembolization, new molecular target therapies (sorafenib, per a example) and symptomatic treatment [3,30].
Palliative management is considered in around 50% of patients with HCC currently diagnosed and the end point of palliation should be an improvement in survival. This can only be demonstrated through randomized controlled trials including large numbers of patients [16].
One of the palliative treatments that has showed improve survival of patients was the chemoembolization [31]. Transcatheter arterial chemoembolization (TACE) is benefic on patient survival when used appropriately in patients with unresectable HCC and preserved liver function; still is an effective therapeutic option for cirrhotic patients with HCC [2,32].
In Brazil, chemoembolization is the most common therapy used for patients with HCC, including those in the early stages; liver resection was sometimes indicated in patients with a high risk of recurrence. The majority of patients with advanced HCC did not have access to new
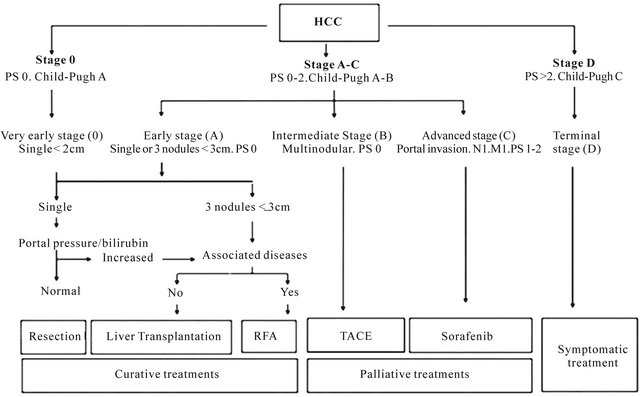
Figure 2. The Barcelona clinic liver cancer staging system for HCC and treatment approach [28]. M = metastasis classification; N = node classification; PS = performance status; RFA = radiofrequency ablation; TACE = transarterial chemoembolization.
molecular target therapies, such as sorafenib [13].
Sorafenib is a small molecule oral that inhibits tumorcell proliferation and tumor angiogenesis and increases the rate of apoptosis in a wide range of tumor models [33,34]. It has been shown in patients with advanced hepatocellular carcinoma that sorafenib prolonged median survival and the time to radiologic progression by nearly 3 months longer for patients treated with sorafenib than for those given placebo, and diarrhea, weight loss, hand-foot skin reaction, and hypophosphatemia were more frequent adverse events [35]. Still, sorafenib neoadjuvant therapy is cost-effective for HCC patients waiting for liver transplantation, particularly for median times for it under 6 months [36].
Other molecular target therapy for HCC has been developed and there are currently many clinical trials evaluating tyrosine kinase inhibitor (TKI) for HCC, including those tested in combination with (e.g., erlotinib) [37] or compared with (e.g., linifanib) sorafenib as a first-line therapy [38]. Although, at the moment, the only drug that has given positive results during phase III trials (SHARP study) is sorafenib [39].
For patients who do not respond or are intolerant to sorafenib, TKIs such as brivanib, everolimus, and monoclonal antibodies (e.g., ramucirumab) are being tested as second-line therapies and the combination of bevacizumab with oxaliplatin and capecitabine demonstrated encouraging efficacy and good tolerability, even in those patients with suboptimal performance status and liver function [38,40].
Recently, a product containing stem cell differentiation stage factors was used for treat patients with advanced stage HCC and showed evidence of benefits due complete tumoral response [41].
The chemotherapy, with single agents or in combination regimens, does not prolong life in HCC [42]. Finally, patients at a terminal stage who have very impaired physical status or tumor progression should receive symptomatic treatment [43].
Patients with low bilirubin, high albumin, no ascites, and high performance status tended to live longer [44]. The survival rate of patients with cirrhosis who develop HCC remains poor, with a 5-year survival rate of 17%. Although different treatment modalities are available, improvement in survival is achieved only if the tumor is diagnosed at an early stage and identification of HCC smaller than 20mm in diameter is associated with longer survival. However, the presence of vascular invasion, even when the tumor is small, is associated with poor prognosis [45].
In untreated HCC patients, the available evidence is sufficient to conclude that the 1-year and 2-year survival is extremely variable, bad performance status, ChildPugh B-C classes, and presence of portal vein thrombosis are associated with a worse prognosis; and the presence of ascites is associated with poor survival in intermediate/advanced BCLC stages [46].
3. Psychosocial Consequences
The patients without therapeutic perspectives of healing, terminally ill, may be very fragile, with immobility, loss of interest in food and beverage intake, as well as weakness and drowsiness. Due to high levels of anxiety, tension and emotions, it is very important that professional team start to develop a structured conduct in order to control symptoms and support patients and their families [19,47].
The World Health Organization profess that cancer diagnosed patients need a psychosocial monitoring in order to evaluate the anxiety and depression, giving support in order to help patient keeping the treatments plans and basic emotional support. In addition, the psychosocial support must be available for the family and employees who treat cancer patients [25].
According to the study performed in Taiwan, the most common symptom seen in HCC patients upon admission to the hospice was pain (75.5%). The majority of patients suffered from abdominal pain, which originated from the enlarged tumor mass and was characterized as dull visceral pain. One-third of the patients experienced bone pain, which was aggravated upon moving due to bone metastasis. Fatigue or weakness, peripheral edema, cachexia, ascites, dyspnea, anorexia, and vomiting were also common complaints [14].
The physical pain in patients with end-stage liver disease is often accompanied by emotional and psychological pain, many times due to incapacity for working to support oneself and one’s family. This leads to significant income decrease, savings become depleted, and they mourn the loss of lifestyle and familiar relationship [47,48].
In addition, the presence of hepatic encephalopathy make them unable to drive, becoming a caregivers dependent, besides suffering from the disease progressing signals such as weight loss and ascites. Finally, patients develop fear and insecurity because of gastrointestinal bleeding, which seems to be out of control [49].
As liver diseases are asymptomatic on the first years, the consciousness of one’s death appears only when complications begin, which unable them to take part of planning and decision taking. Furthermore, psychological symptoms such as depression, anxiety and mood changing may happen alone or come along with hepatic encephalopathy, adding to the patient’s general bad state [14].
One of the most frequently asked questions by patients or their family members in the hospital is “How long do I have?” or “How long does he/she get?” A predictable timing allows them to be well prepared for the final stage of the patient’s life and can also aid physicians in recommending the most appropriate palliative care to each patient [1].
Elisabeth Kübler-Ross proposed a progression of stages by which terminal patients deal with their impending death: from denial, anger, bargaining, depression, and when all goes well, final acceptance. Thus, the depression stage is not considered pathological at the death proximity moment. In this context, an interiorization process is observed, patient is many times debilitated, withdrawn from the loved ones and this fact is not supposed to be understood as rejection, but as a process of preparing for the final death. Still, we must understand the significance of silence, the quietness and, mainly, the welcoming presence. At this moment, there is no need of speaking or doing, but only providing the comforting presence [50].
Nevertheless, unfortunately the hospital routine makes hard the silence and privacy. Therefore, the Palliative Care Program facilitates this type of monitoring at the end of life, especially because they allow families to be present for longer [51].
However, it is frequent very little support when it is most needed, particularly those with chronic hepatitis C or alcoholic cirrhosis who had addictive lifestyles and had hurt and alienated people along the way. In these moments, nurses and social workers can enable open communication between patients and family members, helping them to finish unfinished business and facilitate relationship closure, easing the facing of death [22,24].
The patients more involved in their care were more likely to have surveillance than patients who did not feel involved. Furthermore, they have a high level of concern about HCC, want more information from their physicians and treatment’s decision should be always considered [52]. A Brazilian study have documented that cancer diagnosed patient manifests the desire of being informed by the doctor (96.1% men and 92.6% women) and that the notice also be given to their family (87.7% of men and 84.2% of women). The studied population expects to be informed about therapeutics (82.2%) and a lower percentage wants to participate in the treatment decision (50.8%) [23].
4. Conclusions
The chronic diseases treatment and life quality improvement had resulted in an increase of Brazilian elderly population. Therewith, the increasing demand for a palliative care area is a challenge for the health workers. However, patients with hepatocellular carcinoma in a palliative setting have a poor prognosis despite recent therapeutic progress.
Among the available palliative treatments for patients in end-stage of HCC, sorafenib is the only approved drug still able to increase survival. Enlighten the diagnosis with no way of cure, inform the patient detailed and in an understandable way, and talking about therapeutic options guarantee a doctor patient relationship improvement and more belief on the health team.
The multidisciplinary team will have to begin talking in order to plan the patient and families care, before the end-stage disease. The education for death must be no longer a taboo for the patients, family and health worker, for indeed, it is a life natural process. This is an issue which demands a broad reflection and the involved professionals need to have their palliatives care concepts, thanatology and bioethics clear in order to discuss about how to dealing with prejudice and to exercise effective communication.
NOTES