Efficacy and Safety of Iron Isomaltoside Compared with an Oral Iron Supplement in the Management of Patients with Iron Deficiency Anemia ()
1. Introduction
Anemia is the most prevalent nutritional anemia worldwide, and it is also one of the most concerning public health problems. Anemia refers to a common clinical symptom when the volume of red blood cells in human peripheral blood is decreased below the lower limit of the normal range and cannot adequately supply and deliver oxygen to tissues and organs. Hemoglobin (Hb), red blood cell count (RBC) and hematocrit (Hct) are often used to diagnose anemia in clinical practice, among which Hb is the most commonly used and reliable. The cutoff levels of Hb for diagnosis of anemia depend on age, sex and pregnancy (Table 1) [1] [2] . The samples used in this study were collected from China, so we followed the anemia diagnostic criteria based on the health industry standard for blood cell analysis followed by the People's Republic of China. Anemia is graded as shown in the Table 2. Iron deficiency anemia (IDA) is the most common type of anemia, which is caused by the lack of iron required to produce Hb. Anemia is a public health issue in developing countries, especially for women of childbearing age [3] . According to the release of the “Report on the Status of Nutrition and Chronic Diseases in Chinese Residents (2020)”, the anemia rate of residents aged 18 and above in China is 8.7%, the anemia rate of children and adolescents aged 12 - 17 is 6.6%, the anemia rate of children aged 6 - 11 is 4.4%, and the anemia rate of pregnant women is 13.6%, which is significantly lower than the results released in 2015 [4] , IDA remains a major public health concern in our country [5] . The prevalence of anemia was 15.0% in non-hospitalized and non-pregnant childbearing women from the Chinese Fifth National Health and Nutrition Survey [3] , and the incidence is higher in poor
![]()
Table 1. Hb (g/L) concentrations to diagnose anemia at sea level [1] [2] .
![]()
Table 2. The severity of Anemia [1] .
areas and remote mountainous areas. A study initiated by the school of public health of Fudan University on the disease burden and diagnosis status of IDA in China showed that the medical expenses for patients with chronic diseases complicated by IDA would increase by 30% to 40% [6] . IDA has become a serious health problem that affects people’s physical and mental health. IDA can cause various clinical symptoms, including fatigue, palpitation, impaired physical performance, impaired cognitive function, hair loss, headache, paresthesia, sleeping disorders, female infertility, repeated upper respiratory tract infections, or even serious infections. The concern is that, in pregnant women, IDA is associated with fetal growth retardation, the intellectual development of newborns, and high susceptibility to infections. Anemia is not only a kind of hematological disease but is also associated with sensorineural hearing loss [7] [8] [9] , depression [10] , thyroid dysfunction during pregnancy [11] , fibromyalgia [12] , venous thromboembolism [13] , ischemic stroke [14] , heart failure [15] , cancer [16] , affecting the treatment and prognosis of these diseases. Hence, correcting IDA is of great significance to improving the quality of life of the broad masses of people and reducing the occurrence of adverse events. The treatment of IDA needs to identify and correct the underlying cause and provide medical iron therapy. This paper is a retrospective research project that focuses on the use of novel intravenous iron in patients with IDA and the comparison of novel intravenous iron with oral iron supplementation.
Oral iron supplementation which has been widely used in the treatment of IDA is absorbed from the duodenum to the upper jejunum. Absorption of oral iron is easily affected by gastric acid, food, digestive tract function and infection status, and gastrointestinal issues [17] . It takes a long time to correct anemia [18] , which easily leads to poor patient compliance, and recurrent anemia and affects the quality of life of patients. At the same time, compared with allogeneic blood transfusion, intravenous (IV) iron supplementation can quickly improve Hb, reduce the demand and acute and long-term risks of allogeneic RBC transfusion such as fever, allergic reactions, the transmission of infectious diseases, alloimmunization, iron overload, and immunosuppression for patients who need to quickly improve Hb [19] [20] , and save blood sources to a great extent.
Chinese expert consensus on the application of intravenous iron (2019) [21] showed IV iron is preferred to oral iron application in many cases: Patients unable or unwilling to tolerate gastrointestinal adverse effects of oral iron administration; Patients prefer to replenish iron storage by 1 - 2 visits; Persistent blood loss and exceeding the ability of oral iron agents to meet the needs for iron supplementation; Anatomical or physiological situations affect the absorption of oral iron; Interference with iron metabolism homeostasis by comorbid inflammation; Expected blood loss mid massive (>500 ml) surgery, or iron deficient patients requiring surgery in <6 weeks. Besides, some chemotherapy-induced anemia clinical trials with IV iron monotherapy yielded positive results, which either leads to increased Hb levels, decreased RBC transfusion requirement, or both [22] . Intravenous iron may be more cost-effective for many of the above patients [21] . Although intravenous iron supplementation such as iron sucrose has already appeared, it has not been widely used because of its side effects and high frequency of administration. The main evaluation criteria for the safety of intravenous iron supplementation include the stability of carbohydrates and their compounds, the release of free iron or non-transferrin-bound iron, and the level of immunogenicity. As a new intravenous iron supplementation, iron isomaltoside is a pure linear chemical structure composed of α 1 - 6 linked glucose units, with an average size of 5.2 glucose units and an average molecular weight of 1000 Da. Each isomaltoside pentamer in its matrix contains about 10 iron molecules, which have a strong binding structure and can control and slowly release biologically effective iron to iron-binding protein while the risk of free iron toxicity is very small, which solves the problem that low-dose iron agents such as iron sucrose cannot be given in full dose at one time due to free iron toxicity [23] . Use of a single total dose infusion results in a decreased number of intravenous infusions with a lower cumulative risk for infusion reactions or extravasations, a reduced need for multiple office visits and repeated utilization of medical staff, and increased convenience for physicians and patients [24] . Clinical trials of various iron isomaltoside have been reported, and their effectiveness and good safety have been proven [25] . Iron isomaltoside 1000 was marketed in China in April 2021. So far, there is no observational study on the safety and effectiveness of iron isomaltoside in China. Therefore, this retrospective study aimed to compare the efficacy and safety of treatments with one-time intravenous infusion of iron isomaltoside and oral iron in the management of IDA in patients in China and provide practical experience for the application of iron isomaltoside anhydride in China.
2. Data and Methods
2.1. Research Object
The present study was designed as a monocentric and retrospective survey. This study screened eligible patients and collected relevant data through the hospital’s internal data management system, outpatient cases, test results and telephone follow-up.
99 patients with IDA treated with iron isomaltoside in the Department of Hematology, the Affiliated Hospital of Qingdao University from October 2021 to September 2022 were selected as Iron Isomaltoside 1000 group, at the same time 130 patients with IDA treated with oral iron supplementation were taken as the control group.
Divide the patients who meet the inclusion and exclusion criteria into two groups, Group A: MonoFer 1000 mg was injected intravenously; Group B: NIFEREX 300 Qd was taken orally.
2.2. Inclusion and Exclusion Criteria
Inclusion criteria.
Before treatment, Hb < 120 g/l, ferritin < 13 μg/l.
Age ≥ 18 years old.
Patients in group B were treated without interruption for at least 3 months from the start of treatment.
Patients in Group A or Group B participated in complete post-baseline follow-up.
Patients who did not receive iron therapy within three months prior to treatment
Exclusion criteria.
Non-IDA (such as hemolytic anemia and aplastic anemia).
Severe anemia and extremely severe anemia.
Other forms of iron supplementation, blood transfusion, erythropoietic agent infusion, radiation and chemotherapy during the trial.
This study was retrospective and approved by the hospital Ethics Committee.
2.3. Objectives and Endpoints
The trial was designed with the objectives to compare safety and efficacy of oral iron supplementation to iron isomaltoside in patients with IDA.
The main study indicator in this study was Hb. It takes approximately 2 months of oral iron therapy for Hb to return to normal in patients with IDA, and further iron supplementation is required for insufficient storage iron [1] . To ensure adherence, we included Hb at 3 months of oral iron therapy.
The study duration for an individual patient was approximately 3 months. Iron isomaltoside group consists of 3 visits: baseline examination (baseline and dosing visit), mid-term examination (1-week follow-up), and final examination (1-month follow-up). The oral iron group consists of 3 visits: baseline examination (baseline and dosing visit), mid-term examination (1-month follow-up), and final examination (3-month follow-up). Some patients in intravenous iron group received oral iron supplementation in 1 month. Data were recorded at baseline, 1 week, 1 month, and 3 months. The validity data are Hb, Mean corpuscular volume (MCV), Mean corpuscular hemoglobin (MCH), Mean corpuscular Hemoglobin concentration (MCHC), and Platelet (Plt). The primary efficacy endpoint was the change of Hb levels. The secondary efficacy endpoints included the change of MCV, MCH, MCHC, and Plt levels. The adverse reactions of iron supplementation were recorded. This study is in line with the principles of the Helsinki Declaration.
2.4. Efficacy Evaluation
The levels of biochemical parameters (Hb, MCV, MCH, MCHC, and Plt) were measured at baseline and after treatment.
In the group, the biochemical parameters were compared before, after 1 week, and 1 month of administration of iron isomaltose, and before, after 1 month, and 3 months of administration of oral iron were compared. The groups were graded according to the severity of anemia before medication (Table 1), and the rising level of the biochemical parameters of anemia of different degrees in 1 month after medication was compared.
2.5. Safety Evaluation
Common Terminology Criteria for Adverse Events (CTCAE - Version 5.0) were used to evaluate adverse events (AEs). Serious Adverse Events (SAEs) are defined as CTCAE ≥ grade 3.
2.6. Statistical Analysis
Friedman tests were used for intra-group comparison. Differences except gender between the groups were compared with the Mann-Whitney U test for two independent samples. Gender between the groups was compared by Chi-square test. Statistical analyses were performed using SPSS 26.0 and GraphPad Prism 8.0. P-values < 0.05 were considered statistically significant.
2.7. Outcome
As a clinical observational study, when analyzing the data, this study compared biochemical parameters (Hb, MCV, MCH, MCHC, and Plt) before the application of iron isomaltoside (99 subjects) and within one week and one month after application, and those before application of oral iron (130 subjects) and within 1 and 3 months after application. The inter-group comparison was divided into two groups mild anemia (56 orally administered and 25 intravenously administered) and moderate anemia (74 orally administered and 74 intravenously administered) anemia. The inter-group comparisons of the biochemical parameters were performed at 1 month of dosing.
2.8. Comparison within Groups
According to SPSS 26.0 and Friedman’s test, the intra-group comparisons revealed that the biochemical parameters were significantly improved (P < 0.05) at 1 month after treatment whether in iron isomaltoside or oral iron, except for Plt levels of iron isomaltoside and MCHC levels of oral iron, whether it was iron isomaltoside or oral iron (Figure 1, Table 3 and Table 4).
2.9. Comparison between Groups
There were no significant differences in gender, age and biochemical parameters before treatment (Table 5). The biochemical parameters of iron isomaltose group were significantly improved compared with oral iron group after 1 month of treatment, and the difference was statistically significant (P < 0.05) (Figure 2, Table 6).
2.10. Safety Evaluation
Among patients with IDA treated with iron isomaltoside, there are 5 patients developed rash, which improved after anti-allergy treatment or on their own. There were 2 patients with arthralgia, which improved spontaneously. There
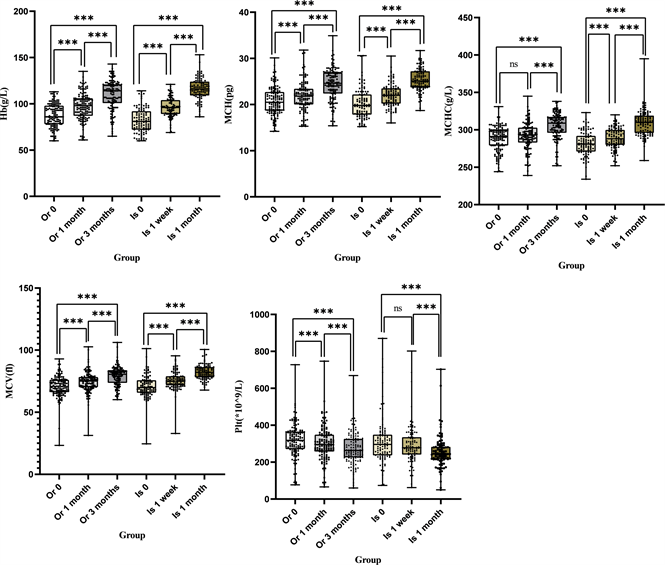
Note: Or 0: the baseline of oral iron, Or 1 month: 1 month after the treatment of the baseline of oral iron, Or 3 months: 3 months after the treatment of the baseline of oral iron, Is 0: the baseline of iron isomaltoside, Is 1 week: 1 week after the treatment of the baseline of iron isomaltoside, Is 1 month: 1 month after the treatment of the baseline of iron isomaltoside. *** means P < 0.05 between two groups, ns * means P ≥ 0.05 between two groups.
Figure 1. The Intra-group Comparison.
![]()
Table 3. Comparison of Clinical Outcomes of IDA Patients in the iron isomaltoside group.
Note: *Means P < 0.05 when compared to baseline, #Means P < 0.05 when compared to 1 week.
![]()
Table 4. Comparison of Clinical Outcomes of IDA Patients in the oral iron group.
Note: *Means P < 0.05 when compared to baseline, #Means P < 0.05 when compared to 1 month.
![]()
Table 5. Basic Clinical Characteristics of IDA Patients in the iron isomaltoside group versus the oral iron group.
were 2 patients with hyperpigmentation of the skin, which improved spontaneously. Dyspnea occurred in 1 person, and improved after anti-allergy treatment. 1 patient suffered anaphylactic shock and recovered after active rescue. No adverse reactions occurred in the remaining patients. The incidence of adverse events of iron isomaltose was about 11.11%.
The most common adverse effects of oral iron were gastrointestinal upset and no SAEs were found (Table 7).
3. Discussion
IDA is an important type of anemia, which can reduce the quality of life and labor ability as a poor prognostic factor for chronic diseases. As such, diagnosis and effective treatment of IDA are of critical importance. For patients attending
![]()
Table 6. Comparison of Clinical Outcomes of IDA Patients in the iron isomaltoside group versus the oral iron group.
the outpatient clinic, the etiology of IDA is routinely actively sought based on iron supplementation. Clinical and preclinical studies on iron sucrose and iron isomaltoside concerning the mode of injection have been relatively mature. Iron isomaltoside became available in China in April 2021, and there are relatively few related studies. This article mainly compares the clinical efficacy and safety of intravenous iron isomaltoside with oral iron supplementation in IDA patients.
Iron isomaltoside is currently approved in more than 30 countries, and clinical trials have shown it to be well tolerated and to improve markers of IDA in patients with chronic kidney disease on dialysis, patients with non-dialysis-dependent patients with chronic kidney disease, chronic heart failure, inflammatory bowel
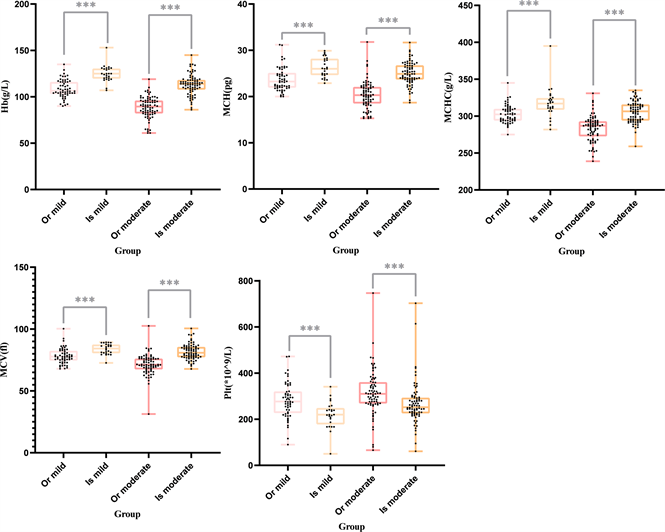
Note: Or mild: the change of oral iron in 1 month in mild anemia, Or moderate: the change of oral iron in 1 month in moderate anemia, Is mild: the change of iron isomaltoside in 1 month in mild anemia, Is moderate: the change of iron isomaltoside in 1 month in moderate anemia. *** means P < 0.05 between two groups, ns * means P ≥ 0.05 between two groups.
Figure 2. The inter-group comparison.
disease, cancer patients, patients undergoing cardiac surgery, and women with postpartum hemorrhage [24] . However, there is a need for studies of iron isomaltoside used in daily practical settings for the treatment of Chinese anemic patients. The objective of this observational study was to investigate the effectiveness, safety, and tolerability of iron isomaltoside in routine practical care of IDA in patients.
Biochemical parameters (Hb, MCV, MCH, MCHC) were included in the study. Hb was used to determine the severity of anemia. MCV, MCH and MCHC were used to determine small cell hypochrome anemia. Ferritin was used to diagnose IDA. In addition, Plt was also included in this study because many studies on IDA suggested that the level of Plt was related to the iron deficiency status [26] [27] [28] . Benjamin found that iron deficiency as well as iron replacement can have a range of effects on platelet production and function, whose possible mechanism is that megakaryocytes share a mutual precursor cell with erythrocytes, and megakaryocytic/erythroid progenitor cells appear to preferentially differentiate toward the production of megakaryocytes in iron deficient states. Besides, thrombocytosis during IDA may be an adaptation to augment hemostasis in anemia related to blood loss [27] . In this study, the decrease of platelet count after treatment was considered to be related to the improvement of iron deficiency status.
Oral iron is a common treatment for IDA. However, in case of side effects, intolerance or inadequate compliance to oral therapy, poor gastrointestinal iron absorption, erythropoietin administration, severe iron deficiency, and chronic blood loss, intravenous iron replacement is indicated [29] . Intravenous iron therapy increases Hb more quickly and reduces blood transfusions, especially in peri-operative patients. The use of intravenous iron is limited due to its adverse reactions and dosing intervals. Compared with traditional iron sucrose, iron isomaltose has the advantages of a more rapid effect and better safety and no allergy test was required. Besides, the risk of serious infusion reaction caused by the iron isomaltose was reduced by 49% compared with the iron sucrose. Moreover, the incidence of compound cardiovascular adverse events caused by the one-time injection of iron isomaltose was significantly lower than that caused by 5 times iron sucrose injection. A randomized trial compared the efficacy of iron isomaltose and iron sucrose in patients with IDA and found that iron isomaltose was more effective than iron sucrose in achieving rapid improvement in Hb [30] . This provides a basis for the application of intravenous iron in China.
At present, the efficacy of iron isomaltose has been less reported in China. A total of 229 patients were enrolled in this study. One-time intravenous infusion of iron isomaltoside was compared with the observation of the effectiveness and safety of oral iron in the treatment of IDA in China, to provide practical experience for the application of iron isomaltoside in China. We found that both iron isomaltoside and oral iron can effectively improve Hb. Iron isomaltoside can more rapidly increase Hb levels through this study and the adverse reactions were mild. The incidence of adverse reactions with iron isomaltoside was similar to that reported in previous literatures.
It has been proposed that hypophosphatemia associated with parenteral iron therapy may be mediated by fibroblast growth factor 23 [31] [32] [33] [34] . Hypophosphatemia is much less common in iron isomaltose-treated patients than in iron carboxymaltose, but it is recommended that phosphate levels be monitored 1 to 2 weeks after iron isomaltoside injection in high-risk patients, such as severe anemia patients requiring repeat dosing, and patients with past vitamin D deficiency and/or hyperparathyroidism. However, Philip A. Kalra et al. believed that iron isomaltoside did not seem to induce hypophosphatemia [35] . This indicator has not been tested in this study. Besides, there are still many shortcomings in this study. This study lacks the data of severe and extremely severe anemia and the detection of ferritin, liver function, kidney function, electrolyte and other test indicators, which needs to be further improved by subsequent prospective studies.
4. Conclusion
In conclusion, both iron isomaltoside and oral iron supplementation were effective in increasing Hb. And high-dose iron isomaltoside can increase Hb effectively and rapidly with a controllable safety.
Author Contributions
ZMW, CXZ, CYW, QS, SLC, WZ, and SLW have jointly designed the research question, prepared the manuscript, and revised it. All authors contributed to the article and approved the submitted version.