1. Introduction
Diabetes is a metabolic disease of various etiologies, characterized by chronic hyperglycemia, accompanied by a disturbance of carbohydrate, lipid and protein metabolism, resulting from a defect in the secretion of insulin or the action of insulin or these two associated abnormalities [1] . Hyperglycemia leads to acute and chronic degenerative metabolic complications. The number of people with diabetes continues to increase every day and this number has increased from 108 million in 1980 to 422 million in 2014. This figure is expected to continue to grow to reach 622 million in 2040 [2] . Many studies have demonstrated that increasing serum levels of total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) and decreasing high-density lipoprotein cholesterol (HDL-C) increase the factors of risk of atherosclerosis [3] . Epidemiological studies have indicated that dyslipidemia and blood clotting disorders are among the most important risk factors for the development of atherosclerotic diseases [4] . Oxidative stress plays a major role in the pathogenesis of various diseases such as diabetes and coronary heart disease. Hydroxyl free radicals have been shown to be responsible for peroxidative damage to lipoproteins present in the blood, which in turn are responsible for the initiation and progression of atherosclerosis [5] . Although there are antidiabetic, antihyperlipidemia and antistress medications that rely on oral antidiabetics or statins. These pharmacological treatments are effective in reducing hyperglycemia, triglycerides, and low-density lipoprotein (LDL), but most patients still experience coronary events despite this treatment. In addition, other studies report undesirable side effects such as the risk of hypoglycemia for oral antidiabetics and myopathy of certain statins [6] . Additionally, the class of fibrates, which are primarily used to treat hypertriglyceridemia and low-density lipoprotein cholesterol (LDL-C), require high doses to show significant effectiveness. Ultimately, hyperglycemia and dyslipidemia, which are the two major components of metabolic syndrome, are also one of the crucial risk factors for cardiovascular diseases [7] . Hence the interest in finding medicinal plants with hypoglycemic, hypolipidemic and antioxidant properties and at least or without side effects. Many medicinal plants have been studied and shown their hypoglycemic, hypolipidemic and antioxidant effects in humans and animals, among them the seeds of B. senegalesis which not only are used as food, but also play a great role in the fight against diabetes and associated diseases in traditional Chadian and Cameroonian medicine [8] [9] [10] . With this in mind, our contribution in the fight against diabetes and associated diseases is to study the antidiabetic and antistress effect of B. senegalesis extract in rats. Hence the objective of this work to evaluate the effect of the aqueous extract of Boscia seeds. senegalensis on hyperglycemia, hyperlipidemia and oxidative stress in an animal model fed a hyperlipidic diet.
2. Methods
2.1. Chemicals
All the chemicals were purchased from Sigma-Aldrich Chemie Gmbh (Munich, Germany); Total cholesterol and total triglyceride were estimated using diagnostic kits (Fortress, UK). HDL was determined using the MONLAB kits (Barcelona, Spain); Atorvastatine MICRO LABS LIMITED (Bengaluru, India).
2.2. Experimental Animals
Male wistar rats aged 6 to 8 weeks and weighing between 145 and 195 g were purchased from the Animal Shop of the Faculty of Sciences of the University of Ngaoundéré. Rats were housed in a standard environment with 23˚C ± 1˚C, 55% - 65% humidity, and under a 12 h light/12 h dark cycle. Only male rats were used for this study in order to avoid the changes in menstrual cycle of females which may affect the results, such the excretion of estrogens known to protect the onset of obesity and diabetes. During acclimation (7 days), rats had free access to tap water and a normal standard diet (3810 kcal/kg) consisting of fish meal (20%), soybean oil (5%), vitamin complex (1%), mineral salts. (5%), sucrose (5%), cellulose (5%) and corn flour (59%), daily (Table 1). After one week of adaptation, hyperlipidemia and diabetes were induced in forty normal male rats divided into 8 groups of 5 rats each: two normal control groups which received a normal standard diet (3810 kcal/kg), and six groups were subjected to a high-fat diet (5310 Kcal/kg) consisting of fish meal (20%), soybean oil (5%), coconut oil (25%), cholesterol (1%), vitamin complex (1%), mineral salts (5%), sucrose (5%) and corn flour (38%). All groups of animals were fed in this way for 8 weeks. The standard, high-fat diet used in this study was previously formulated in our laboratory and was successfully tested to induce hyperlipidemia and diabetes in rats [11] . At the end of the 8 weeks induction period, one group of rats fed with high-fat diet and one group fed with standard normal diet were sacrifice to verify whether hyperlipidemia, hyperglycemia and oxidative stress had been achieved. After the induction of, hyperlipidemia, hyperglycemia and oxidative stress, the remaining six groups of animals were treated from the following eight weeks with either distilled water for the normal control and negative control groups, atorvastatin for the positive control group or with B. senegalensis extract (125, 250 and 500 mg/kg) for the control test groups. During the induction period, the body weight of all animals were taken weekly. During the treatment period, in addition to body weight, the fasting blood glucose of all animals were
![]()
Table 1. Formulation of standard diet and High-fat diet [11] .
taken weekly. The Oral Glucose Tolerance Test (OGTT) were taken at the end of the treatment. At the end of eight week of treatment, rats were sacrificed after anaesthetized using diethylic ether, the blood was collected by cardiac puncture and used for analysis of biochemical and oxidative stress parameters. The liver, kidney, testicle, heart and adipose tissues were equally removed and weighed to assess the relative weight and for the production of homogenates after grinding for analysis of oxidative stress parameters. All in vivo tests on animals were carried out in accordance with European Union guidelines for the protection of animals (EEC Council 86/609) [12] .
2.3. Sampling and Production of Boscia senegalensis Flour
Seeds of B. senegalensis were collected around the University of Ndjamena, Chad in November 2019. Thespecies was identified and a voucher specimen was deposited at the Facha Veterinary and Zootechnical Laboratory (N˚E 1344). Dried mature seeds were manually cleaned to remove defective seeds. The dry seeds were then ground into a fine powder using an electric grinder (Culatti, Polymix, France) fitted with a sieve with a diameter of 800 µm, then sealed in polyethylene bags and stored at room temperature (25˚C ± 2˚C) for subsequent analyses.
2.4. Preparation of the Aqueous Extraction of Boscia senegalensis Powder
Preliminary studies were carried out in the laboratory to research different therapeutic doses by studying the acute toxicity of B. senegalensis extract on rats [13] . An aqueous extract of B. senegalensis was produced from the seed flour with distilled water. The production conditions of this decoction were determined in previous studies to maximize the flour mass/water volume ratio, phenolic content and DPPH scavenging activity and to minimize the glycemic index; the extraction time, temperature and flour mass/water volume ratio were 10 min, 55˚C and 3/10 g/mL respectively [13] . After incubation, the sample was centrifuged at 3000 rpm for 10 min at 25˚C. The supernatant was collected in a 100 ml volumetric glass container and stored at −4˚C in the refrigerator.
2.5. Qualitative Analysis on Phytochemical Constituents
Characterization of tannins: To characterize the tannins, 2 g of plant powder were boiled in 20 ml of distilled water in a water bath. The aqueous extract obtained was filtered using filter paper (ϕ: 0.1 mm) on a conical flask. 3 drops of 0.1% FeCl3 were added to the filtered sample and the brownish green or blue black color observed showed the presence of tannins [14] .
2.5.1. Characterization of Flavonoids
Flavonoids in the extract were investigated using the alkaline reagent test. For this test, in 0.5 mL of extract, add 4 drops of sodium hydroxide. The appearance of the intense yellow color which disappears after addition of diluted HCl indicates the presence of flavonoids [15] .
2.5.2. Polyphenol Detection
In 0.5 mL of the plant extract, 5 mL of distilled water was added in a test tube. 3 drops of 5% ferric chloride were added to the mixture and the appearance of a bluish black testified the presence of polyphenols [15] .
2.5.3. Detection of Anthocyanidins
0.5 ml of extract was added to 10% (v/v) aqueous KOH and the observation of red color suggested the presence of anthocyanidins [16] .
2.5.4. Glycoside Detection
4 ml of plant extract was dried down to 2 ml. then 1 ml of ammonium hydroxide was added to it and the mixture was shaken. The appearance of the darling red color indicates the presence of glycosides [17] .
2.5.5. Alkaloids
Alkaloids in the extract were tested for by mixing 4 ml of the extract with 2 ml of 1% HCl and heating gently. After adding Mayer’s and Wagner’s reagents to the mixture the turbidity of the resulting precipitate was taken as evidence of the presence of alkaloids [17] .
2.5.6. Testing for Anthraquinons
It was carried out using the potash test [18] . Thus, 0.5 ml of extract was mixed with 0.1 ml of aqueous KOH solution (10%, w/v). The formation of red color indicates the presence of anthraquinons.
2.5.7. Detection of Saponins
Saponins were detected using the Froth test [19] . In a test tube containing 0.1 ml of extract, 1.5 ml of distilled water was added. The solution was shaken vigorously and allowed to stand for 20 min. The persistence of a 1 cm foam for 10 min explained the presence of saponins.
2.6. Fasting Glycaemia Estimation and Oral Glucose Tolerance Test (OGTT)
At the end of each week, the fasting blood glucose of each animal was taken for 8 weeks, the oral glucose tolerance test (OGTT) was performed at the end of the treatment. All blood glucose levels (fasting blood sugar and OGTT) were taken using the “ONE TOUCH Ultra” glucometer (Lifescan, U.S.A). Blood glucose was taken by cutting the posterior end of the tail vein of each animal and a drop of blood was taken from the test end of a strip mounted on a glucometer for direct reading of blood glucose [20] . To evaluate the OGTT test, blood sugar was taken at 0 min before administration of D-glucose (3 g/kg) and then at 30, 60 and 120 min after taking D-glucose in each animal [20] . Glucose concentrations were expressed as mg/mL of blood
2.7. Evaluation of Biochemical Parameters
The blood serum from each animal was collected after coagulation at room temperature and then centrifugation at 1500 rpm for 20 min at 25˚C. Total cholesterol and triglycerides were measured using diagnostic kits (Fortress, UK). The HDLc level was assessed using the MONLAB kits. VLDLC and LDLC were calculated using the Friedewald equation namely: VLDLC = serum triglycerides/5 while LDLC = serum total cholesterol – (VLDLC + HDLC). The activities of ALT and AST transaminases were determined following the method of [21] . Creatinine was assessed following the procedure described by [22] . The results of these parameters were expressed in mg/dL.
2.8. Body Weight, Relative Weight of Organs and Biochemical Estimation in Tissue Homogenates
Changes in body weight for each animal were measured every weekend. The liver, kidneys, testicles, heart and abdominal fat were removed and freed of adhering tissues, rinsed with ice-cold normal saline (0.9%) then drained in to a clean tissue and weighed on a sensitive weighing. The relative weight of each organ was calculated as follows:
. (1)
After calculating the relative weight of each organ, 1 g of tissue was ground in 10 ml of 0.2 M Tris-HCl. The homogenate was filtered then centrifuged at 1500 rpm for 20 min at 25˚C. The obtained supernatant was used for the estimation of oxidative stress parameters: superoxide dismutase (SOD), catalase (CAT) and malondialdehyde (MDA) [23] . The MDA level was expressed using the Beer-Lambert formula as follows:
(2).
CAT and SOD activities expressed as:
. (3)
2.9. Statistical Analysis
The data are presented as mean ± standard deviation (SD) from five animals in each group. All data were statistically analyzed using one-way analysis of variance (ANOVA) followed by post-hoc test (Student-Newman-Keuls) using Graph Pad Prism software version 5.03. A value of p < 0.05 was considered statistically significant.
3. Results
3.1. Different Compounds Presented in the Boscia senegalensis Extract
Phytochemical studies carried out on the extract of B. senegalensis seeds reported the presence of bioactive compounds such as: Alkaloids, saponins, tannins, glycosides, flavonoids, anthraquinones and polyphenols (Table 2).
3.2. Effect of Extract on Body Weight and Relative Weight of Internal Organs
The induction of diabetes in rats was carried out for 8 weeks (from week 1 to week 8). The animals that became diabetic were treated for 8 weeks (from week 9 to week 16). After disease induction (end of week 8), the average weight of rats fed the hyperlipidic diet was significantly (p < 0.001) higher than that observed in normal rats. During the treatment of the animals, rats in the normal control and negative control groups had a significant increase (p < 0.001) compared to those which received either the reference drug atorvastatin or the extract of B. senegalensis (125 , 250 and 500 mg/kg). In the normal control group, the body weight increased from 149 ± 4 to 249.5 ± 5 g while the animals in the negative control group, the body weight increased from 149 ± 17 to 401.3 ± 5 g (Figure 1). The weight increases in the two groups of rats were respectively 32.08% compared to animals treated with 500 mg/kg of B. senegalensis and 60.84% compared to animals in the normal control group.
The relative weight of the organs examined after 8 weeks of treatment showed significant differences between the groups (Table 3). The relative weights of the testis, kidney, and heart did not change significantly with treatment, while the relative weights of the liver and abdominal fat were significantly altered. In fact, relative liver weight was significantly (p < 0.001) decreased in the groups receiving B. senegalensis extract and atorvastatin, due to the absence of fatty deposition in these organs. Additionally, the relative weight of abdominal fat was 318% higher in the negative control group than in the normal control group. In the groups of hyperlipidemic control rats given atorvastatin or B. senegalensis extract, no significant increase was observed in relative weight.
Overall, administration of B. senegalensis extract, whatever the dose, or atorvastatin 10 mg/kg for 8 weeks reduced abdominal fat identical to that of animals in the normal control group (1.04 ± 0.01 g/100 g).
![]()
Table 2. Different compounds present in the Boscia senegalensis extract.
+: Presence of compound.
![]()
Figure 1. Effect of B. senegalensis decoction on the body weight in hyperlipidemic diet induced hyperlipidemic on rats. Values were expressed as mean ± SD; n = 5 rats in each group; ap < 0.001: compared to the normal control; Ator: Atorvastatine 10 mg/kg; NC: Normal control group; HC: hyperlipidemic control group; B. s: Boscia senegalensis (125, 250, and 500 mg /kg); Ator: Atorvatatine 10 mg/kg.
![]()
Table 3. Kidney, heart, testicle, liver and abdominal fat weight of hyperlipidemic diet induced hyperlipidemic rats after 8 weeks treatment.
Values were expressed as mean ± SD for 5 rats in each group; *p < 0, 05; ***p < 0.001: compared to the normal control; ##p < 0.01; ###p < 0.001: compared to the Hyperlipidemic control group; Ator: Atorvastatine 10 mg/kg; N C: Normal control group; H C: hyperlipidemiccontrl group; B.s: Boscia senegalensis (125, 250, and 500 mg /kg); ATOR: Atorvatatine 10 mg/kg.
3.3. Effect of B. senegalensis Extracton the Fasting Blood Glucose and the Oral Glucose Tolerance Test (OGTT)
Fasting blood sugar in rats shows that in the normal control group, blood sugar did not change significantly. It went from 69.4 ± 1.14 in the first week of treatment to 69 ± 0.54 g/dL at the end of the 8th week. In the negative control (diabetic) group, fasting blood glucose increased significantly (p < 0.001) from 128.8 ± 3.96 mg/dL in the first week of treatment to 144.6 ± 1.51 mg/dL at the end of week 8. Diabetic rats given either atorvastatin or B. senegalensis extract showed a significant reduction (p < 0.001) in fasting blood glucose at the end of week 8. At a dose of 500 mg/kg, fasting blood sugar increased by 129.4 ± 6.4 mg/dL at the end of the first week of treatment to 66.0 ± 5.3 mg/dL at the end of treatment thus displaying a value not significantly different from that of the normal control group (Figure 2).
The cellular response of rats receiving an oral glucose overload is presented below (Figure 3). The administration of a glucose overload (3 g/kg) orally to the animals induced a significant increase in blood glucose levels up to a peak followed by a reduction regardless of the treatment administered. In all cases, the peak was reached after 30 minutes after glucose administration, followed by the gradual decrease between 30 and 120 minutes. In rats in the normal control group, a reduction of 50.6% was observed compared to 19.0% in the diabetic control groups. In the groups of animals that received atorvastatin, the reduction was 54%, while in the groups that received B. senealensis extract (250 and 500 mg/kg), the reductions were 50% and 51% respectively.
3.4. Effect of B. senegalensis Extract on Blood Biochemical Parameters
The effect of B. senegalensis decoction on some serum biochemical parameters is shown in Figure 4. Creatinine levels in normal and diabetic rats were not significantly different. It had a mean value of 5.2 ± 0.15 mg/L in animals in the normal control group, 5.48 ± 0.39 in animals in the negative control group. The activity of the ALT enzyme was 46.92 ± 0.98 in normal animals, 46.26 ± 0.76 U/L in animals in the negative control group and 48.14 ± 0.96 U/L. in animals treated with B. senegalensis extract at 500 mg/kg.
The effects of B. senegalensis extract on obese rats are similar to those of atorvastatin. Compared with the above biochemical parameters, the lipid profile was significantly influenced by atorvastatin and B. senegalensis extract. Hyperlipidemic control rats in the negative control group are characterized by significantly higher levels of triglycerides, total and LDL cholesterol, and lower levels of HDL cholesterol. Administration of B. senegalensis extract to obese rats induced a significant reduction in triglycerides, LDL and total cholesterol (Figure 4). In this regard, the variations in lipid profile parameters were 71.4 ± 1.1 mg/dL for triglycerides, 91.4 ± 2.0 mg/dL for total cholesterol, 36.9 ± 1.5 mg/dL for LDLc and 42.6 ± 1.81 mg/dL for HDLC in animals in the negative control group versus 47.4 ± 0.9; 46.4 ± 0.9; 17.4 ± 1.17 and 14.2 ± 1.81 respectively for
![]()
Figure 2. Effect of B. senegalensis decoction on the fasting blood glucose on obese rats. NC: Normal control; HC: hyperlipidemic control; B. s.: Boscia senegalensis (125, 250, and 500 mg /kg); Ator: Atorvatatine 10 mg/kg. Values were expressed as mean ± SD for 5 rats in each group; cp < 0.001: compared to the normal control: *p < 0.001 compared to the Hyperlipidemic control group.
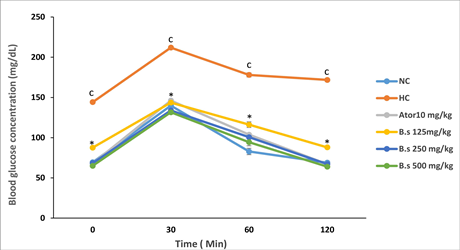
Figue 3. Effect of Boscia senegalensis decoction on the oral glucose tolerance test in obese rats. NC: Normal control group; HC: hyperlipidemic control group; B. s: Boscia senegalensis (125, 250, and 500 mg /kg); Ator: Atorvatatine 10 mg/kg. Values were expressed as mean ± SD for 5 rats in each group; cp < 0.001: compared to the normal control: *p < 0.001 compared to the Hyperlipidemic control group.
these same parameters in animals in the positive control group (atorvastatin). In animals receiving 500 mg/kg of B. senegalensis, the lipid profile parameter values were: 36.0 ± 2.6 mg/dL for triglycerides, 50.8 ± 1.4 mg/dL for total cholesterol 19 .3 ± 1.4 mg/dL for LDLc and 45.4 ± 2.1 mg/dL for HDLc.
The effect of B. senegalensis was dose-dependent, with the 500 mg/kg dose having the highest effect and the 125 mg/kg dose having the lowest effect.
3.5. Effect of B. senegalensis Decoction on Tissue Antioxidant Parameters
Malondialdehyde is an intermediate product of lipid peroxidation, protein and
![]()
Figure 4. Effect of B. senegalensis extract on Blood biochemical parameters. NC: Normal control group; CH: hyperlipidemic control group; B. s: Boscia senegalensis (125, 250, and 500 mg /kg); Ator: Atorvatatine 10 mg/kg Values were expressed as mean ± SD for 5 rats in each group; cp < 0.001: compared to the normal control: δp < 0.001 compared to the Hyperlipidemic control group.
carbohydrate oxidation, and its level in organs increases during stress and chronic diseases. Oxidation is initiated by highly reactive oxygen radicals which, under normal body conditions, are removed by endogenous enzymes such as catalase (CAT) and superoxide dismutase (SOD). Increased MDA and reduced CAT and SOD activities in blood and organs are synonymous with increased oxidation. The variations of MDA in the liver, kidney and serum of rats subjected to different treatment protocols are shown as follows (Table 4).
MDA levels in blood, liver and kidney of hyperlipidemic control rats were significantly (p < 0.001) higher than in normal rats. The values obtained in these organs were respectively 1.36 ± 0.10 µM; 1.37± 0.20 µM and 1.39 ± 0.01 µM for normal rats, compared to 2.64 ± 0.01 µM, 3.2 ± 0.01 µM and 3.81 ± 0.00 µM for hyperlipidemic control rats. Treatment of hyperlipidemic control rats with B. senegalensis extract significantly (p < 0.001) dose-dependently reduced MDA levels in all organs. MDA levels in blood, liver and kidney were 1.56 ± 0.01 µM; 1.48 ± 0.00 µM and 1.53 ± 0.00 µM for the 125 mg/kg dose in these organs, 1.44 ± 0.01 µM; 1.39 ± 0.00 µM and 1.42 ± 0.00 µM for the dose of 250 mg/kg and 1.34 ± 0.00 µM; 1.36 ± 0.00 and 1.34 ± 0.01 for the 500 mg/kg dose. Similarly, administration of atorvastatin 10 mg/kg resulted in a significant (p < 0.001) reduction in MDA in blood, liver and kidney compared to hyperlipidemic control animals.
The results on SOD and CAT activities revealed that hyperlipidemic control rats had lower enzyme activities than normal rats and those of rats treated with B. senegalensis extract. The variations observed for SOD in diabetic animals compared to normal animals were 7.82 ± 0.1 Unit/mg compared to 11.66 ± 0.3 Unit/mg in blood; 6.02 ± 0.1 Unit/mg versus 10.45 ± 0.1 Unit/mg in the liver and 7.22 ± 0.1 Unit/mg versus 11.35 ± 0.1. Unit/mg in the kidney. For CAT, the variations were 34.42 ± 0.01 Unit/mg versus 45.7 ± 0.06 Unit/mg in blood, 36.24 ± 0.02 Unit/mg versus 48.03 ± 0.01 in the liver and 33.26 ± 0.3 Unit/mg versus
![]()
Table 4. Superoxide dismutase (SOD), Catalase (CAT) and Malondialdehyde (MDA), in Liver, kidney and serum.
Values were expressed as mean ± SD for 5 rats in each group; *p < 0. 05; **p < 0.01;***p < 0.001: compared with normal control; ###p < 0.001: compared to the obesity group; N C: Normal control group; Ator: Atorvastatine 10 mg/kg; H C: hyperlipidemic control group; B. s: Boscia senegalensis (125, 250, and 500 mg /kg); ATOR: Atorvatatine 10mg/kg; MDA: malondialdehyde (µM); SOD: superoxyde dismutase (µU/g); CAT: catalase (U/g).
45.35 ± 0.01 Unit/mg in the kidney. In hyperlipidemic control rats treated with B. senegalensis extract, SOD and CAT activities were significantly increased in blood, liver and kidney. In this regard, the activities of SOD at the dose 500 mg/kg of B. senegalensis extract were 11.7 ± 0.1; 10.5 ± 0.7 and 11.4 ± 0.3 respectively in blood, liver and kidney while CAT activities were 46.0 ± 0.1; 48.7 ± 0.6 Unit/mg and 45.4 ±. 0.2 Unit/mg respectively in the same organs.
4. Discussion
Wistar rats fed a high-fat diet (HFD) were found to accumulate excess fat in their abdomens. Compared to normal rats, rats fed a high-fat diet weighed 1.5 times more than normal. A 1.7-fold increase in weight was also reported in genetically susceptible mice fed a high-fat diet, which delineates obesity with components associated with metabolic syndrome [24] . The fat composition of our normal diet and the HFD were 5% and 31%, respectively, lower than the 10% and 60% reported by these authors. However our HFD contains 1% cholesterol which was not present in their diet. High-fat diets are generally known as high-energy diets, which inhibit the hypophagic action of leptin and therefore increase food intake. In this study, we found no significant difference in weight gain between obese and normal rats fed the standard diet for 8 weeks. This suggests that obesity does not have a significant effect on food consumption. Our result is in agreement with the study by [25] . Which shows a reduction in body weight in obese diabetic rats fed dietary fiber. We found in this study that the administration of B. senegalensis extract induced a reduction in body weight, similar to that of atorvastatin, a reference drug used in the treatment of obesity. This indicates the effectiveness of the extract in reducing weight. The decrease in the relative weight of abdominal fat observed in B. senegalensis extract or atorvastatin administered to rats suggests an increase in lipid catabolism activity. The regulation of fat metabolism depends on several hormones including leptin, which has been the subject of numerous studies. Leptin has a direct autocrine and paracrine mode of action on lipid metabolism. It appears to intervene in fatty acid metabolism through the concentration of enzymes such as inhibition of carboxylase in adipocytes, an enzyme essential for the synthesis of fatty acids and the conversion of glucose to fatty acids and the storage of energy in the form of triglycerides. This leads to the activation of fatty acid oxidation, key even for the reduction of tissue lipids and the insulin-sensitizing effect of leptin, making the hormone considered antisteatotic [26] . B. senegalensis extract can also act directly on certain key enzymes of lipid metabolism or the inflammatory reaction. In this regard, some studies on B. senegalensis revealed the presence of bioactive components such as flavonoids and alkaloids [13] [27] . These bioactive components have been shown to increase the activity of enzymes involved in the regulation of serum lipids [28] . In addition to these targeted effects, phenolic compounds and a number of other components, such as diterpenes and melanoidins, have been shown to possess antioxidant properties and inhibit the production of inflammatory mediators and enhance inflammatory processes [29] .
The mechanism of action of B. senegalensis extract has not yet been studied and need to be investigated. One of the most important changes occurring in obese rats was the increase glycaemia and the limited ability to preventincrease in post prandial blood glucose. The blood glucose level increased constantly in obese ratseven though they were submitted to normal food regime. This is characteristic of the inhibition effect of fat accumulation on insulin secretion [30] . This study showed that this goes increasing with time. According to these authors, fat accumulation rapidly and specifically reduces the central actions of insulin and leptin [30] . In rats obese rats fed B. senegalensis extractor atorvastatin, not only the glycaemia was normalized, but also the oral glucose tolerance in glucose loaded rats was improved. These observations denoted the hypoglycemic property of B. senegalensis extract. The extract may act indirectly by reducing the fat level which negatively affect insulin secretion, ordirectly by stimulating the secretion of insulin, improving the carbohydrate catabolism or decreasing intestinal glucose absorption [31] . We found a significant linear correlation (r = −0.93; p < 0.001) between the reduction in weight gain, due to B. senegalensis extract or atorvastatin administration, and the blood glucose level, suggesting the negative role of fat accumulation and the glycaemia. In order to understand the mechanism of hypoglycemic action of B. senegalensis extract, its effect on insulin secretion in normal and diabetic rats needs to be investigated. In addition its effect on the glucose intestinal absorption needs to be investigated as it has been reported that B. senegalensis is an inhibitor of glucose absorption, a methylglucosinolate called glucocapparin. The activity of enzymes in glucose catabolism also needs to be investigated since B. senegalensis contains some vitamins (A, B1, B2, PP and C) and minerals (Si, P, Ca, Mg and K) which may act as modulators [32] [33] .
The present study and many others showed that hyperglycemia is associated to dyslipidemia. This observation corroborates the studies which reported increased hyperglycemia, insulin resistance and hyperlipidemia in rats fed high fat diet [34] . We found that B. senegalensis extract and atorvastatin improved the hypertriglyceridemia and hypercholesterolemia seen in obese rats. In particular the blood LDL and total cholesterol, and triglyceride contents were significantly decreased while the HDL cholesterol was significantly increased. An increase blood cholesterol and triglycerides level is a consequence of fat accumulation in adipocytes and hepatocytes. Many studies reported hypercholesterolemia and hypertriglyceremia in rat fed high cholesterol/fat diet [35] . Due to the fact that LDL cholesterol is associated with a higher risk for cardiovascular disease, intervention to reduce LDL becomes relevant. By lowering the LDL and total cholesterol and triglycerides, and increasing the HDL cholesterol levels in blood, B. senegalensis show a beneficial effect, in a manner similar to atorvastatin. Atorvastatin reduces cholesterol levels by slowing down the production of cholesterol and increasing the liver’s ability to remove LDL cholesterol from the blood [36] . Many seeds extracts such as Vitiated faba, Vigna unguiculata and Pisum sativum, Triticum aestivum, Xylopia aethiopicahave shown similar therapeutic benefits [37] [38] and [39] . The regulation of many enzymes activities have been suggested mechanism through which extracts play role in the reduction of cholesterol level. In this respect, increasing the activity of lecithin cholesterol acyltransférase (LCAT) increases the hydrolysis of lecithin of lipoproteins and produces ester of cholesterol towards the excretion of cholesterol into the bile [40] . In addition inactivation of hydroxyl-methyl Glutaryl –Coenzyme A reductase (HMG-Coa reductase) activity lead to reduction of cholesterol synthesis in live. [41] [42] . Noted a rise in the activity of the LCAT and an inhibition of HMG-CoA reductase, in the rats treated with Symplocos cochinchinensis extract. In addition, [43] observed a reduction of triglycerides level in rats submitted to Vitiated faba extract and explain this by a lowering of the activities of enzymes acylCoA synthetase and the acetylCoA carboxylase. The action of B. senegalensis extract on activity of all these enzymes need to be investigated. In concordance with literature, we observed that high fat diets increase oxidative stress parameters in plasma and several tissues such as liver, heart [44] . In particular oxidation of LDL cholesterol cytotoxicity is implicated in the process leading to atherosclerosis. The uptake of oxidized LDL by macrophage and endothelial cells is cytotoxic leading to foam cell formation, damage and smooth muscle formation in pathological processes [45] . Malondialdehyde (MDA) is one of the most evaluated intermediary component of subtract oxidation used to determine the extent of cellular oxidation [45] . In addition, the levels of antioxidant enzymes superoxide dismutase (SOD) and catalase (CAT), the first line of cellular defense against oxidant, decrease beyond a certain concentration of oxygenated species in the organism [46] . It is therefore interesting to inhibit oxidation of lipids in general and LDL in particular to prevent atherosclerosis. We found in this study that the MDA level increased and the SOD and CAT activities decreased in rat fed high-fat-cholesterol diet; interestedly, the levels of these oxidative markers diminished (MDA) or increased (SOD and CAT) when administered B. senegalensis extract. These results strongly suggest that B. senegalensis extract is potentially applicable to prevent atherosclerosis. It has been shown in several studies such as the present one that hypercholesterolemic subjects developed oxidative stress [44] . It has equally been shown that plants extract (i.e. from artichoke leaves, Hibiscus sabdariffa, Bitter melon) rich in natural antioxidants has a cholesterol lowering effect, reduced the oxidation of LDL-VLDL and consequently decreased the formation of MDA [37] [44] [47] . Some natural antioxidants including α and γ tocopherol, β carotene and retinal stearate and polyphenols may be involved in this mechanism by entering the structure of lipoproteins and protecting LDL from oxidative oxidation [46] . However no study has been interested in the characterization of antioxidants in B. senegalensis seeds, and this need to be investigated.
Increase in MDA is a consequence of disequilibrium of the balance oxidant/antioxidant in favor of oxidant [48] . In this respect we found in hyperlipidemic rats significant decrease in the antioxidant enzymes SOD and CAT concomitant with increase in blood MDA level. SOD catalyzes the dismutation of the anion superoxyde
to form hydrogen peroxide H2O2 and thus to reduce the toxic effects due to this free radical or other radicals which derive from the secondary reactions [47] . CAT catalyzes the reduction of hydrogen peroxides in H2O and O2 and protects cells from the H2O2 which are very reactive [48] . If the H2O2 produced by SOD is not broken up immediately, it can involve the lipidic peroxidation [49] . The reduction of the activity of SOD and CAT observed in the blood, liver and the kidneys of the hyperlipidemic controlrats could be due to the negative effect of radicals either on enzyme activity or enzyme concentration. In fact glycation of SOD has been shown to be involved in enzyme inactivation which contributes to the aggravation of the oxidative damage caused by the overproduction of the anion superoxyde (
) and of the hydroxyls radicals (OH•) [50] .
5. Conclusion
In Africa and more particularly in Cameroon, diabetes and obesity pose a real alarming problem due to changes in lifestyle. The results of our study indicate the effectiveness of the extract of B. senegalensis on the cellular mechanisms which control the reduction of the glucose level, the loss of body weight and the metabolism of the lipids in hyperlipidemic control rats. The extract of B. senegalensis reduces the lipid peroxidation and improves the state of oxidative stress which provides the proof that the use of this plant could protect the liver, heart, brain and testicular against pathologies dregs with the noxious effects of the reactive species of oxygen.
Acknowledgements
We thank the Food Biophysics and Biochemistry Laboratory of the Department of Food Science and Nutrition, National School of Agro-industrial Sciences, University of Ngaoundere Cameroon Which allowed us to carry out the tests for the production of this article.
Authors’ Contributions
All authors participated in the design experiment, with N. N.Y. and S.S.D. as the main leaders. F.D. and E.B.D carried out the experiments; F.D; A.H.M and E.B.D participated in animal experiments. N.N.Y and F.D analyzed and interpreted the data; F.D. wrote the diary. All authors read and approved the final manuscript.
Funding
Not applicable.
Data Availability
The data used in this research can be made available by the corresponding author upon a reasonable request.
Ethics Approval and Consent to Participate
All authors declare that all guidelines for the care and use of laboratory animals have been made in accordance with the European Union guidelines for animal protection (EEC Council 86/609) and have been reviewed and approved by the Institutional Ethics Committee of the University of Ngaoundéré.
Consent for Publication
Not applicable.