Analysis and Assessment on the Heavy Metals in a Severely Degraded Subtropical Red Soil Region ()
1. Introduction
Soil provides the necessary environmental conditions for the growth and development of plants, and is also affected by the environment [1] [2] . Some metal elements in the soil are important nutrients necessary for plants. Its lack or accumulation will affect the original chemical balance of the soil, destroy the balanced environment of the soil, and cause physiological pathological changes of plants [3] [4] [5] . Excessive accumulation of soil metal elements can be transported to rivers through surface water, leading to deterioration of the quality of the ecological environment [6] . Forests are plants, animals and their integration with the environment. They are very important land and natural resources, and are important for soil and water conservation, biodiversity conservation, climate regulation and the maintenance of sustainable ecosystem development [7] . With the continuous development of human science and technology, human activities have had a certain impact on the forest ecosystem.
In recent years, some scholars have studied soil heavy metal pollution, including heavy metal pollution source analysis and risk assessment. However, it mainly focuses on farmland soils and urban soils. There are few reports on the properties and spatial distribution of heavy metal elements in degraded forest soils. There are also a few studies on the correlation between heavy metal elements in forest soils and other soil factors. Some studies have shown that effective content of soil heavy metal elements has a certain correlation with different forest land types [8] [9] [10] . The total amount of heavy metals is the storage of heavy metal elements, indicating the potential supply capacity of the soil. Generally, the higher the total amount of heavy metals, the higher the effective state content [11] . Some studies have found that the total amount of trace elements in soil has a significant correlation with soil parent material, and is also affected by soil weathering and leaching effects [12] [13] . In addition, heavy metal elements are more difficult to decompose, which is likely to cause high accumulation of forest surface soil and plant roots [14] , which in turn affects the structure and function of forest soil ecosystems, and can destroy ecological balance through circulation [15] [16] . Therefore, the spatial distribution characteristics of different heavy metal elements in subtropical degraded red soil forests were studied, and the pollution was evaluated. The research results provided basic data for sustainable development and ecological environment protection.
2. Materials and Methods
2.1. Site
The site is located at the subtropical monsoon humid climate. It is located in Taihe County (26˚44'N, 115˚04'E) in central Jiangxi Province, China. The annual average temperature is 18.6˚C, the average annual precipitation is 1726 mm, and the rainfall is concentrated from April to June, the heat and dry season was from July to September. The soil forming matter is red clay with a large amount of surface gravel, which belongs to the typical red soil low hilly land. Before the 1980s, due to long-term human disturbance, the native evergreen broad-leaved forest no longer existed, and the vegetation coverage was greatly reduced, leaving only 30% and uneven distribution. In the 1990s, artificial transformation was carried out, and P. massoniana, S. superb, L. formosana and other tree species were used for afforestation, and a large area of diverse vegetation restoration types was established. At present, the current vegetation coverage has reached more than 90%, the forest phase structure is relatively complete, the forest microclimate has been formed, and the ecological, economic and social effects are good. The heavy metal content of soil in this area is studied to investigate the distribution of heavy metals in the subtropical forests and to evaluate the pollution.
2.2. Experimental Design and Sampling
The sampling time was January 2015. In the study area, the five types of plantations of P. massoniana, S. superb, L. formosana, and P. massoniana × L. formosana, P. massoniana × S. superba were randomly selected. For each type of object, select 3 plots with a size of 20 m × 20 m. Use a five-point sampling method for each plot. Drill the 0 - 10 cm surface soil with a soil drill, mix it, remove impurities, and remove it by quadruple method. Some of them are packed in aseptic bags, and the remaining samples are sealed and taken back to the laboratory for relevant soil nutrient analysis.
2.3. Methods
2.3.1. Sample Determination Method
The sample is air-dried in a cool place, and impurities such as soil animals, stones and plant residues are removed, ground with an agate mortar, passed through a 2 mm sieve, and bagged for use. The total carbon and total nitrogen content of the soil were determined by elemental analyzer. The pH value was determined by a 1:2.5 aqueous solution and a pH meter. The water content was obtained after drying the fresh soil sample at 105˚C for 48 h. The heavy metal content of the soil was determined by aqua regia-perchloric acid digestion-atomic absorption spectrometry [17] . The results of soil properties were in Table 1.
2.3.2. Pollution Assessment Criteria and Methods [18]
The Class II soil evaluation standards were used to evaluation criteria for heavy metal pollution in domestic forest soils. This study uses the national soil environmental quality class II standard for evaluation. It adopts the single factor pollution index and Nemerow comprehensive pollution index method commonly used
![]()
Table 1. Soil basic properties under different forest types.
at home and abroad. The soil quality of different forest land was evaluated.
The formula for calculating the single factor pollution index is:
Note: Pi is the environmental quality index of the pollutant i in the soil, Ci is the measured mass fraction (mg/kg) of the pollutant i, and Si is the evaluation standard of the pollutant i.
The formula for calculating the comprehensive pollution index is:
Note: (Ci/Si) max is the maximum pollution index in soil pollutants, and (Ci/Si) ave is the average of the pollution index in soil pollutants.
When the single factor pollution index Pi ≤ 0.6, the pollution level is Grade I, the pollution degree is uncontaminated; when 0.6 < Pi ≤ 1.0, the pollution level is Grade II, the pollution degree is early warning; when 1.0 < Pi ≤ 1.5, the pollution level is III. Level, the degree of pollution is mild pollution; when 1.5 < Pi ≤ 2.0, the pollution level is grade IV, the pollution degree is moderate pollution; when 2.0 < Pi ≤ 3.0, the pollution level is V grade, the pollution degree is heavy pollution; Pi > at 3.0, the pollution level is Grade VI, and the pollution level is severely polluted. For the comprehensive pollution index, the pollution degree is safe when PN ≤ 0.7; the pollution level is the warning level when 0.7 < PN ≤ 1; the pollution degree is light pollution when 1 < PN ≤ 2; the pollution degree when 2 < PN ≤ 3 for medium pollution; when PN > 3, the degree of pollution is heavy pollution.
2.3.3. Statistical Analysis
One-way ANOVA, followed by Fisher’s least significant difference (LSD) test, was employed to examine the effect of different tree species on soil properties. The normality of all data was checked before ANOVA. Pearson’s correlation coefficient was used to assess the relationship between soil heavy content. All statistical analyses were performed using SPSS 17.0 software (SPSS Inc., USA).
3. Results
3.1. Distribution Characteristics of Heavy Metals in Different Forest Soil
The soil metals in different forests have the highest Cr content, all of which are between 80 - 180 mg/kg and the lowest Cu content, all below 80 mg/kg; between different forests, The content of Cr in P. massoniana × S. superba was the highest, which was 169.14 mg/kg, and it was significantly higher than other forest stands (Table 2). The pure Cr content of P. massoniana forest was 93.19 mg/kg, the difference between the content of L. formosana, P. massoniana and L. formosana was not significant, and the difference between the content of S. superba and them was significant. There was no significant difference in soil Cu content
![]()
Table 2. The heavy metal content of soil in different forest land
between different forests (Figure 1). The Cu content of P. massoniana × S. superba in soil is 78.55 mg/kg and the highest, and the content of Cu in the P. massoniana is the lowest, is 64.67 mg/kg. The soil copper content of several forests is P. massoniana < S. superba < P. massoniana × L. formosana < L. formosana < P. massoniana × S. superba. The content of cadmium of P. massoniana × S. superba is the highest, 102.54 mg/kg, is significantly higher than the other forests. The content of P. massoniana Zn is the lowest, 73.71 Mg/kg. The difference in Zn content between the other forests was not significant (Figure 1).
3.2. Pollution Assessment of Heavy Metals in Different Forest Soils
The single factor pollution index is calculated using the national soil en vironmental quality standard (Class II) as the evaluation standard. The single factor pollution index of Cr content in P. massoniana, S. superba, L. formosana, P. massoniana × L. formosana is 0.62, 0.85, 0.66, 0.79, which is early warning grade, P. massoniana × S. superba Cr. The single factor pollution index was the highest (1.13), which was mildly polluted; the single factor pollution index of Cu content in P. massoniana pure forest, S. superba, L. formosana, massoniana × L. formosana was 1.29, 1.30, 1.38, 1.36, which is a mild pollution. The single factor pollution index of Cu content in P. massoniana × S. superba is the highest (1.57), which is moderate pollution. The single factor pollution index of soil Zn content in some forest land is below 0.6, which is uncontaminated. The Cr content of the
![]()
Figure 1. The heavy metal content of soil in different forest land.
single pollution grade is the early warning level, the Cu content is mildly polluted, and the Zn content is uncontaminated. The pure forest of P. massoniana of the comprehensive pollution index was the lowest in the five different forest types, and the comprehensive pollution index was 1.06. The comprehensive pollution index of P. massoniana × S. superba was the highest, is 1.34. The comprehensive pollution index of the five forest types was light degree of pollution (Table 3).
3.3. Correlation between Soil Nutrients and Heavy Metal Elements
Pearson correlation analysis was adopted on soil nutrients and heavy metal elements. There was a significant negative correlation between soil pH and soil Cr, Cu and Zn content, indicating that the concentrated metal in the study area was greatly affected by soil pH. There was a significant positive correlation between soil Cr content and Cu content. The correlation coefficient was 0.556. There was a significant positive correlation between Cu and Zn, Cr and Zn. The correlation coefficients were 0.828 and 0.840 (Table 4), respectively. These results indicated that the soil metal content in the study area is highly complex and related to each other. The statistics of soil carbon, nitrogen and phosphorus content and soil metal did not significant levels, indicating that the correlation between soil metal
![]()
Table 3. Soil pollution index of different forest lands.
![]()
Table 4. Pearson correlation analysis of the concentrations of soil nutrient and element in different forest.
Note: ** and * indicated significance at P < 0.01, and 0.05, respectively.
content and soil carbon, nitrogen and phosphorus content was not significant.
3.4. Analysis of the Regulation Path of Heavy Metal Activity in Different Forest Types
The soil C, P and water content have significant positive effects on heavy metal activity, and the direct path coefficients are 0.8, 0.69 and 0.74, respectively (Figure 2). The combination of soil N, C and N and soil water content showed a significant positive effect on metal activity, but weakened the effect of N on heavy metals under soil pH. The path coefficient was -0.14, in the role of water content. The effect on heavy metals is significantly enhanced; soil C exhibits a positive effect on metals with a path coefficient of 0.8, but under the influence of soil pH, the effect of soil C on soil metal activity is weakened; pH also shows heavy metals. The negative effect, through the influence of soil water content, significantly enhanced the effect on heavy metal activity with a path coefficient of 0.74. So, there indirect effects can not be ignored in addition to the direct effects between the factors. Therefore, the direct and indirect effects between the various
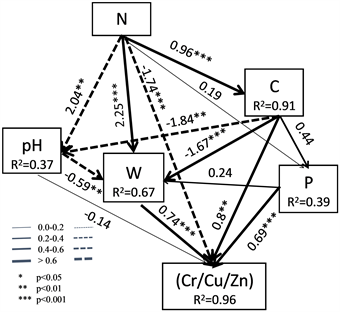
Note: The solid lines indicate positive path coefficients and dashed lines indicate negative path coefficients, R2 values represent the proportion of the variance explained for each endogenous variable.
Figure 2. The structural equation model showing the hypothesized causal relationships between each variable.
influencing factors ultimately affect the activity of heavy metals through the cascade effect.
4. Discussion
Fossil fuel combustion produces a large amount of Cr [19] , which burns into the forest system with atmospheric deposition. In addition, the metal elements such as Cr and Cu in air pollution mainly come from the mining of minerals, the discharge of industrial waste, and the waste generated by the chemical industry [5] . Our results showed that the highest content of Cr in different forest soils in this study was P. massoniana × S. superba, the content of which was 169.14 mg/kg, which exceeded the national soil environmental quality standard (Class II), indicating the change of soil environment of P. massoniana × S. superba. The accumulation of Cr was promoted, the single factor pollution index of Cr reached a mild pollution level, and the Cr content of the other four forest soils did not exceed the national soil environmental quality standard (Class II), and the pure Cr content of P. massoniana forest was the lowest, 93.19 mg/kg, indicating that the mixed ability of P. massoniana × S. superba for soil three kinds of metal elements is greater than other forest types. The single factor pollution index of S. superba forest was only lower than that of P. massoniana × S. superba, indicating that the S. superba forest was the main cause of soil Cr accumulation [20] . Except for P. massoniana × S. superba, the Cr content of other forest stands showed early warning level, and the single factor pollution level of overall Cr content was early warning level. The reason may be the small environment produced by different forest types [21] , and excessive accumulation of heavy metals in the soil will lead to a decrease in most microbial biomass indicators in the soil and a decrease in soil enzyme activity [22] . Zn is mainly derived from traffic pollution and is called traffic pollution element [23] . The automobile exhaust contains a certain amount of Zn, which is discharged into the atmosphere, and the metal elements in the atmosphere enter the forest ecosystem with atmospheric precipitation and sedimentation. In this study, the soil Zn content was the highest in the soil of P. massoniana × S. superba, the content value was 102.54 mg/kg, and the lowest content in the pure P. massoniana forest was 73.71 mg/kg. Exceeding the national soil environmental quality standard (Class II), it indicates that most of the soil metal Zn is derived from the parent material and a small amount is derived from atmospheric deposition [21] . The soil Cu content of P. massoniana × S. superba was the highest in different forests, the content was 78.55 mg/kg, and the pure Cu content of P. massoniana forest was the lowest, the content was 64.67 mg/kg. The soil Cu content of each forest land was P. massoniana < S. superba < P. massoniana × L. formosana < L. formosana < P. massoniana × S. superba, and the soil Cu content of each forest land is higher than the national soil environmental quality standard (Class II); the content of Cr in P. massoniana × S. superba was higher than that in soil. The content of Cr and Zn in the soil of S. superba was second only to the P. massoniana and S. superba, indicating that the accumulation of Cr and Zn of P. massoniana × S. superba was mainly affected by S. superba. The effect of S. superba on the change of soil environment promoted the accumulation of Cr and Zn. The decomposition of Cr and Zn in S. superba forest was weak and the absorption capacity was strong. The coefficients of variation of Cr and Zn in P. massoniana × S. superba were 6.34 and 6.52, respectively, indicating that the two elements have similar degrees of variation, and the external influences are consistent. It can be inferred that the pollution sources are the same [24] .
The single factor pollution index of Cu content in different forest soils was greater than 1, reaching a mild pollution level, but the difference in Cu content between different forests was not significant, indicating that forest type had little effect on soil metal Cu content; studies showed that minerals in Jiangxi Province The high content of Cu in the middle may cause the Cu content in the soil background value of Jiangxi Province to be higher than most of the rest of the country [25] . The soil Zn content in several forest land types is at the pollution level. The Zn in air pollution mainly comes from automobile exhaust emissions, indicating that the forest system in this region is less affected by human automobile exhaust pollution.
Studies have shown that soil heavy metals are mainly derived from soil parent materials, and soil heavy metal content and soil physical and chemical properties are consistent with changes [26] . In this study, except for soil pH, there was no significant correlation between heavy metal elements and soil physicochemical properties such as carbon, nitrogen and phosphorus, indicating that metal accumulation in soil may be caused by human factors, not from soil parent materials. There was a significant correlation between the three heavy metals, indicating that the soil was affected by human factors [24] . Therefore, the activity of heavy metals is determined by the combined effects of various ecological factors.
5. Conclusions
1) The contents of Cr, Cu and Zn in P. massoniana × S. superba were the highest, indicating that the accumulation of heavy metals in this forest type was higher than that in other forest types.
2) The content of Cu in each forest type exceeds the soil environmental quality standard. The Cr content of P. massoniana × S. superba exceeds the soil environmental quality standard, indicating that the content of Cu in different forest types and the content of Cr in P. massoniana × S. superba is to some extent. There has been accumulation. The comprehensive pollution levels of different forest types reached a mild pollution level, indicating that the forest soil in the area was affected by a certain degree of human activities.
3) There was a significant negative correlation between soil metal content of Cr, Cu and Zn and pH, indicating that the accumulation of metal elements was related to soil acidity and alkalinity. There was no significant difference between the three metal contents and soil carbon, nitrogen and phosphorus. Correlation shows that the level of soil metal content is not derived from the influence of soil parent material. There is a significant positive relationship between the three metal contents, indicating that the sources of accumulation of these metal elements are the same or similar. Path analysis showed that the direct and indirect effects between the influencing factors ultimately affected the activity of heavy metals through the cascade effect.
Acknowledgements
Funding: The work was funded by National Natural Science Foundation of China (32260297), Natural Science Foundation of Jiangxi Province (20224ACB205003). Key Laboratory of Soil Erosion and Control of Jiangxi Province (2021SKTR05) and Key Laboratory of Poyang Lake Wetland and Watershed Research of Jiangxi Normal University (PK2021002). The authors take this opportunity to thank all for support extended for the research.