Soil Physico-Chemical Properties and Different Altitudes Affect Arbuscular Mycorrhizal Fungi Abundance and Colonization in Cacao Plantations of Cameroon ()
1. Introduction
The cacao tree is a tropical perennial crop of the Malvaceae family [1] . It is mainly cultivated for its bean which is a source of income for many farmers in developed countries. In Cameroon, production was estimated at 295,000 tonnes for the 2021-2022 cacao season [2] . The Cameroonian cacao plantations are extended over an area of about 400,000 ha for an overall yield of 0.4 t/ha, which is low compared to the potential yield which is estimated at 3 to 5 t/ha [3] . This poor yield can be explained by several constraints related to diseases, pests and soil fertility [4] [5] . In addition, climate change contributes to the emergence of new pathologies and drought stress [6] . Improving cacao production is therefore a constant challenge for farmers and this impacts the position of cacao as a strategic crop for the economy of the country. Strategies to improve cacao production include the use of chemical inputs and the screening of tolerant genotypes to biotic and abiotic stresses [7] [8] . However, these strategies remain limited due to the high cost of chemical inputs and the scarcity of tolerant genotypes. One of the current issues is to build agricultural systems that can be sustainable and resilient while preserving ecological balances. In this case, special attention should be given to ecosystem resources through the development of ecological technologies easily available with positive effect on the environment.
Arbuscular mycorrhizal fungi (AMF) are known to be able to establish a beneficial association with about 85% of vascular plants [9] . They play a fundamental role in the maintaining and restoration of soil fertility. Their implication in plant drought tolerance, plant protection against certain telluric pathogens, plant biofortification and in the improvement of hydro-mineral nutrition of plants have been widely developed by several authors [10] [11] [12] [13] . Environmental factors such as altitude, plant communities, soil physicochemical properties and climatic factors influence AMF communities [14] [15] [16] . Some authors have shown that AMF community diversity decreases with increased elevation in temperate climate zones [17] .
Cacao forms an association with AMF communities [18] [19] and selected species of these AMF significantly improve the overall growth of cacao seedlings [20] . In Cameroon, few studies have been made on cacao endogenous AMF as well as factors that can predict their diversity and abundance according to soil properties and altitude. Generally, studies on the interaction between AMF and cacao focus only on the efficiency of the AMF, and sometimes the AMF strains used for effectiveness tests are exogenous to the cacao rhizosphere [21] [22] [23] . It is necessary to determine the environmental variables affecting the cacao endogenous AMF to improve cacao culture. This study aims at characterising cacao plantation soils from three agroecological zones of Cameroon and evaluates the relationship between the endogenous AMF of their rhizosphere, the soil physicochemical characteristics and altitude variations.
2. Material and methods
2.1. Field Site Description
Agricultural production in Cameroon is divided into five agroecological zones. For this study, three agro-ecological zones were selected due to their high productivity in cacao (Figure 1). These areas are the western highlands zone also known as zone III (altitude: 1500 - 2500 m; annual average temperature: 20.64˚C; mean annual precipitation: 3080.5 mm); the mono-modal forest zone or zone IV (altitude: 0 - 4100 m; mean annual average temperature: 24˚C; mean annual precipitation: 4163.5 mm) and the agro-ecological zone with bimodal
![]()
Figure 1. General presentation of localities under study.
rainfall or zone V (altitude: 400 - 1000 m; mean annual temperature: 24.4˚C; mean annual precipitation: 2456.8 mm). The sampling locations in the selected agroecological zone are listed in Table 1, while Figure 1 provides a general overview of the study sites.
2.2. Soil Sampling
Soil samples were collected from field locality of about 5 years old. Sampling was done following the method described by Huang & Cares (2004) [24] . From a cacao tree identified and geo-located in the field using the “GPS essential” application, two concentric circles one from the first three-meter radius, and the second from the six-meter radius were delineated using a tape. On the first circle, four equidistant elementary samples were collected while eight were collected from the second circle. The sampling was done in the stratum from 0 to 20 cm depth under a cacao tree using an auger. Excavation realised on both circles was put into a bucket of 10 L and homogenised in order to constitute a composite sample. In each field, three samples were collected. A total of 78 samples were collected from 9 localities, nine samples per locality except in Melong where, due to sociological constraints in accessing plantations, only six samples were collected. One part of the sample was dried for physicochemical analyses and the other was kept in the refrigerator for microscopic analyses.
2.3. Soil Physico-Chemical Analyses
Soil texture was determined using the Robinson-Kôhn pipette method based on Stokes’ law [25] . Total soil nitrogen (N) was determined by the Kjeldahl method [26] . Soil organic carbon (OC) was determined according to Walkey & Black method using potassium dichromate [27] , and soil organic matter (OM) was then determined from the organic carbon using the formula: OM (%) = Organic carbon (%) × 1724. Available phosphorus (Pavl) was evaluated using Bray II acid solution followed by colorimetric assay with ammonium molybdenum blue with reading at 665 nm [27] . Exchangeable cations (Na+, K+, Ca2+, Mg2+) were determined according to the Schollenberger method by leaching 2.5 g of soil with 100 ml of a 1 M ammonium acetate solution buffered at pH 7. Cation exchange capacity (CEC) was determined using ammonium acetate at pH 7 [27] . Soil acidity (pH) was measured using a “Hanna Instruments” (270 George Washington Hwy; Smithfield, RI 02917, USA) brand pH meter in an aqueous soil extract at the ratio 1:2.5 (ISO 10390 standard). The assessment of the quantity of nitrogen, organic matter, the C/N ratio, available phosphorus, sum of exchangeable cations (SEC), CEC and pH in the soil were based on the standard scale [28] [29] .
2.4. Trapping Culture
AMF spore trapping was done in the green house using sorghum as trap plant. For this purpose, 2.1 kg of substrate was prepared by measuring 700 g of soil in
![]()
Table 1. Geographic position of samples.
The sample codes were designed taking into account the subdivision name followed by the field/sample number followed by its number in the field (from 1 to 3).
each of the three samples collected per field and mixed before being introduced into a 2.5 L bucket. According to the number of samples collected, 26 buckets containing composite soil samples were obtained and used as substrate for trapping. Three seeds previously sterilized in 10% (v/v) sodium hypochlorite for one minute and rinsed with distilled water, were sowed in each substrate. Plants were not supplied with any fertiliser. Plants were checked for sporulation and mycorrhizal colonization after 3 months.
2.5. Isolation and Quantification of AMF Spores
The evaluation of AMF was done in soil samples collected from the localities following trapping in sorghum plant cultivated in soil collected from the localities as described by Morton (1993) [30] . AMF spores were extracted using wet sieving and the decanting method [31] . For this purpose, 50 g of air-dried soil was dispersed in 200 ml of water in a 500 ml beaker. The solution was allowed to settle for decantation for about 30 seconds. The supernatant was decanted through sieves of 500 µm, 250 µm, 125 µm, and 62.5 µm arranged in that order. Four repetitions were made for each sample. The content of the 500 µm sieve, generally composed of debris was discarded. The contents of the three others sieves were collected using a squeeze bottle with demineralized water and they were rinsed in a 50 mL beaker. A suspension of 10 mL was finally obtained for each sample. AMF spores were counted using a dissecting microscope according to INVAM (2023) [32] and expressed as the number of spores per gram of soil.
2.6. Determination of AMF Root Colonization
The determination of AMF root colonization in trapping plant was made in fresh fine roots of sorghum. The root samples were bleached in 10% KOH for 15 min at 80˚C and rinsed with distilled water by acidification in 1% HCl for 10 min at 80˚C. The observation of root colonization was made after staining with 0.05% trypan blue [33] . The percentage and the intensity of AMF root colonization were determined by microscopic observation in 1 cm of stained root fragments according to Trouvelot et al. (1986) [34] . For each sample, 60 root fragments were observed.
2.7. Evaluation of the Correlation between Soil Physico-Chemical Properties, Altitude and AMF Spore Density
Three Principal Component Analysis were performed to evaluate the correlation between soil physico-chemical properties, altitude/agroecological zones and AMF spore density. The first PCA encompassed soil chemical properties, altitude and AMF. Here the samples were grouped by their rate of available phosphorus according to the scale defined by Calvet & Villemin (1986) [28] . The second PCA accounted for soil physical properties, altitude and AMF and the samples were grouped by altitude; three altitude classes were defined, the low altitude class [0 m; 300 m] medium altitude class [301 m; 600 m] and the high altitude class (above 601 m of altitude). The last was conducted with assumptions that the soil properties had not changed during the trapping; therefore the third PCA analysis was made with soil physico-chemical properties, altitude; AMF density and the frequency of mycorrhizal colonization.
2.8. Data Analysis
“The MultiPointTriangle_v1” application was used to determine the different textural classes of the samples (https://www.nrcs.usda.gov/sites/default/files/2022-11/MultiPointTriangle_v1.xlsm). Statistical analyses where perform using R 4.3.0 software. Data were subjected to the one-way analysis of variance (ANOVA) with the Scott-Knott’s HSD test (p-values < 0.05) used to compare mean values of soil variable and AMF variable if the assumptions of normality and homogeneity were satisfied; if not, the Kruskal-Wallis test (p-values < 0.05) was used as an alternative to compare the mean values of soils and AMF variables.
3. Results
3.1. Soil Physico-Chemical Properties
3.1.1. Soil Texture
The arrangement of the soil samples in the textural triangle reveals three main soil groups. Soils with a sandy-loam texture, soils with a sandy-clay-loam texture and soils with a clay-loam texture (Figure 2). Ntui (24, 25 and 26), Bokito (21, 22 and 23), Melong (13 and 14) and Mbanga (10, 11 and 12) have homogeneous samples belonging to the sandy-loam group. The Binguela locality (18, 19 and 20) shows similar homogeneity, but in the silty clay group. Contrarily, localities such as Bafang (1, 2 and 3) and Bassamba (4, 5 and 6) on the one hand, and Tonga (7, 8 and 9) and Penja (15, 16 and 17) on the other, have soil samples distributed between the sandy loam and sandy clay loam groups for the former, and the sandy clay loam and clay loam groups for the latter (Figure 2). All agroecological
![]()
Figure 2. Soil samples texture classes. The figures on the textural triangle refer to the different fields, 1; 2 and 3 for fields from Bafang; 4; 5 and 6 for fields from Bassamba; 7; 8 and 9 for fields from Tonga; 10; 11 and 12 for fields from Mbanga; 13 and 14 for fields from Melong; 15; 16 and 17 for fields from Penja; 18; 19 and 20 for fields from Binguela; 21; 22 and 23 for fields from Bokito; 24; 25 and 26 for fields from Ntui.
zones have a very high sand fraction, i.e. 69.67% (Bafang), 75.5% (Mbanga), 71.5% (Bokito) for the western highlands, the monomodal zone and the bimodal zone respectively (Figure 3). The proportions of sand are up to eight times higher than those of clay (75.5% vs. 9.33%, Mbanga) and almost five times higher than the proportion of silt (75.5% vs. 17.17%, Mbanga), except in the localities of Tonga (western highlands), Penja (monomodal zone) and Binguela (bimodal zone), where this difference is greatly reduced. For these localities, the distribution of sand/ clay/silt is: 44.42%/31.5%/24.08%; 48%/28%/24% and 41.5%/32%/26.5% respectively.
3.1.2. Soil Chemical Properties
Cacao soil chemical characteristics showed that, the highest nitrogen rate (0.46%; p < 0.05, Scott-Knott test) was recorded in Melong in the monomodal zone while Tonga in the Western highlands displayed the lowest rate (0.13%). The rate of
![]() Diffrent letters indicate that there aresignificant differences between the values of the parameter measured in the different localities (Student-Newmans-Keuls, p < 0.05).
Diffrent letters indicate that there aresignificant differences between the values of the parameter measured in the different localities (Student-Newmans-Keuls, p < 0.05).
Figure 3. Ponderal distribution of different particles size according to the study sites.
nitrogen varied from 0.13% to 0.39% in the Western highlands; from 0.20% to 0.46% in the monomodal zone and from 0.20% to 0.38% in the bimodal zone. Carbon/nitrogen ratio indicated that very high discriminative ability was observed in the Tonga locality (C/N: 16.19) in the Western highlands. Soil organic matter composition revealed that all samples are above the standard average rate with significant difference between locations. The significantly higher rates of organic matter were recorded in Bokito (6.14%) in the bimodal zone. Soil P concentration varied from 16.66 to 21.04 ppm in the Western higlands; from 56.96 to 70.57 ppm in the monomodal zone and from 7.57 to 23.51 in the bimodal zone. The highest soil P concentration (70.57 ppm) was found in Melong situated in the monomodal zone and the lowest (7.57 ppm) was found in Bokito situated in the bimodal zone (Table 2). The average level of the exchangeable bases Na+, and Mg2+ was not significantly different between the localities. However, rates of K+ and Ca2+ were significantly different between the localities. Bafang and Bassamba with 1.25 meq/100g each, showed highest rates of K+ while Binguela with 0.20 meq/100g showed lowest rates. The cation exchange capacity (CEC) ranged from 11.71 to 17.49 meq/100g in the Western higlands; from 12.14 to 18.06 meq/100g in the monomodal zone and from 11.48 to 17.05 meq/100g in the bimodal zone. Soil pH level indicated that the soil from Tonga situated in Western highlands was neutral (pH = 6.67), and soils of other study localities were acidic with the lowest pH (4.75) level recorded in Melong locality (Table 2).
3.1.3. Hierarchical Classification of Soils from the Studies of the Agroecological Zones Using Physico-Chemical Properties
Cluster analysis grouped soil samples into two classes (Figure 4). The first class divided in two sub-classes, encompassed on the one hand soils from monomodal
![]()
Table 2. Soil chemical parameters locality per agroecological zones.
The Values with diffrent upper case letter indicate that there is significant difference between the value of the parameter measured in the different localities (Kruskal-Wallis’s test, p < 0.05). The Values with the different lower case indicate that there is a significant difference between the value of the parameter measured in the different localities (Scott-Knott test, p < 0.05).
rainfall locations, namely the soil from Penja, Mbanga and Melong, and on the other hand, soil from Binguela (Bimodal agroecological zone) and Tonga (western higlands agroecological zone). The second group was split into two sub-classes; the first sub-class concerned soil from bimodal rainfall locations, Bokito and Ntui and soil from the western higlands agroecological zone, Bafang. The second sub-class included soil from Bassamba located in the western highlands zone. The correlation matrix showed that within the first group, soil from the monomodal zone are strongly correlated. In the second group, the soil from
![]()
Figure 4. Hierarchical classification of soils from the different study areas using soil physico-chemical properties.
Bafang is closer to that of the bimodal agroecological zone than the soils of the locality of Bassamba. In addition, the Bassamba samples strongly correlated with the soils of Ntui and weakly to those of Bokito.
3.1.4. AMF Spore Abundance
Before trapping
The ranges of AMF spore density varied from 0.26 to 0.98 spore/g soil in the Western highlands; from 0.21 to 0.72 spore/g soil in the monomodal zone and from 0.31 to 1.03 spore/g soil in the bimodal zone. The highest spore density was found in Ntui, Tonga and Melong localities with a record of 1.03, 0.98 and 0.72 spore/g soil respectively (Figure 5). The lowest rate of spore density was recorded in Bafang (0.26 spore/g soil) and Penja (0.21 spore/g soil).
After Trapping
AMF spore density varied from 0.68 to 1.01 spore/g soil in the Western highlands; from 0.57 to 0.76 spore/g soil in the monomodal zone and from 0.46 to 1.03 spore/g soil in the bimodal zone. AMF spore density increased in soil sample from Bafang (from 0.21 to 0.68 spore/g soil), Bassamba (0.74 to 0.98 spore/g of soil), Tonga (0.98 to 1.01 spore/g of soil), Mbanga (0.31 to 0.76 spore/g of soil) and Penja (0.21 to 0.53 spore/g of soil). However, in other soil samples, the spore density reduced namely in soil samples from Melong (from 0.72 to 0.57 spore/g of soil), Binguela (0.58 to 0.46 spore/g of soil), Bokito (0.65 to 0.63 spore/g of soil) and Ntui (1.03 to 0.57 spore/g of soil). The significantly higher spore density (1.01 spore/g soil, Kruskal-Wallis test, p < 0.05) after trapping was recorded in samples where soil from the Tonga area were used as substrate; and lowest spore density was found in samples from Binguela (0.46 spore/g of soil).
![]()
Figure 5. Relative abundance of AMF spores by study areas (a): before trapping; (b): after trapping. Different letters refer to significant differences between AMF spore density (Kruskal-Wallis test, p < 0.05).
3.1.5. Arbuscular Mycorrhizal Fungi Roots Colonization
Several mycorrhizal structures were observed in stained roots with trypan blue, including extraradical hyphae, intraradical hyphae, vesicles and arbuscules (Figure 6). Total AMF root colonization rates were significantly different between localities. Frequency of mycorrhization varied from 27.78% to 63.89% in the Western highlands; from 63.89% to 66.67% in the monomodal zone and from 46.11% to 86.11% in the bimodal zone. Bokito recorded the highest significant (p < 0.05, Scott-Knott test) mycorrhizal frequency (86.11%) while the lowest mycorrhizal frequency (27.78%) was observed with the sample from Bafang. In the western highland zone, the highest rates of root colonization parameters were recorded with samples from Bassamba (F = 63.89%; M = 20.55%; m = 26.13%) and the lowest rates were recorded with the samples from Bafang (F = 42.78%; M = 7.09%; m = 11.90%). No significant difference was found in the frequency of mycorhization in the monomodal agroecological zone (Table 3). The lowest rates of mycorrhizal activity in the bimodal agroecological zone were observed with samples from the Ntui locality (F = 46.11%; M = 9.17%; m = 16.74%) and the highest were observed with sample from Bokito (F = 86.11%; M = 26.50%; m = 30.7.97%).
3.1.6. Correlation between Agroecological Zones, Soil Properties, AMF Spore Density and Root Colonization by the Principal Component Analysis
The principal component analysis (PCA) based on soil properties, agroecological zones and AMF spore density showed differentiation between the localities (Figure 7). The first two dimensions of the PCA accounted for 57.2% of the total variation. The first dimension which explained 37.9% of the data variation was strongly positively correlated with CEC (r2 = 0.91; p < 0.01), total nitrogen (r2 =
![]()
Figure 6. Different AMF structures observed in sorghum roots using sampling soils as inoculums.
![]()
Table 3. Arbuscular mycorrhizal fungi roots colonization per localities.
Frequency of mycorrhization (F %). Intensity of root cortex colonization (M %). Intensity of colonization within individual Mycorrhizal roots (m %). Different low case letters refer to significant difference between the mean of the considering parameters (Scott-Knott test, p < 0.05).
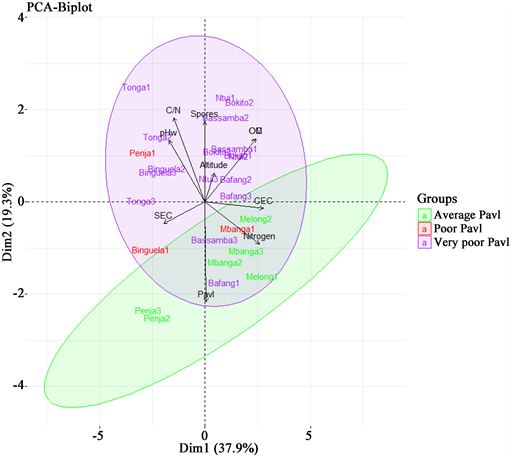
Samples were color-coded according to the rate of available phosphorus (Pavl). Carbon/Nitrogen ratio (C/N); pH water (pHw); Sum of Exchangeable Cations (SEC); Organic Matter (OM), Organic Carbon (OC); Cation Exchange Capacity (CEC); Soil organic Nitrogen (Nitrogen); AMF Spores density before trapping (Spores).
Figure 7. PCA analysis with soil chemical parameters, altitude and AMF spore density before trapping.
0.84; p < 0.01); organic carbon (r2 = 0.79; p < 0.01) and organic matter (r2 = 0.79; p < 0.01). However; The SEC (r2 = −0.64; p < 0.01) and pH (r2 = −0.56; p < 0.01) showed a high and negative effect on this first dimension. The second dimension accounts for 19.3% of the total variation of the data; this dimension is negatively affected by the available phosphorus (r2 = −0.71, p < 0.01) but positively affected by the C/N ration (r2 = 0.60; p < 0.01) and the density of spores before trapping (r2 = 0.57; p < 0.01). According to the PCA, samples from Bassamba (Bassamba 1 and 2) Ntui (Ntui 1 and 2) Bokito (Bokito 1) with high AMF spore density and low rates of available phosphorus are opposed along the second dimension to samples from Penja (Penja 2 and 3) and Mbanga (Mbanga 1, 2 and 3) characterised by lowest AMF spore density and high rates of available phosphorus. However, samples from Melong with a high rate of available phosphorus, high AMF spore density and low pH values are opposed along the first dimension by pH levels to samples from Tonga which has high rates of AMF spore density and high pH values.
The PCA analysis based on the physical soil properties, altitude and AMF spore density showed differentiation between the 26 soil samples studied (Figure 8).
![]() Samples were color-coded according to their altitude. Soil sand content (Sand); soil clay content (Clay); soil slit content (Slit); AMF Spore density before trapping (Spores).
Samples were color-coded according to their altitude. Soil sand content (Sand); soil clay content (Clay); soil slit content (Slit); AMF Spore density before trapping (Spores).
Figure 8. PCA analysis with soil physical parameters, altitude and AMF spore density before trapping.
The first two dimensions of analyses express 75.6% of the total information. The first dimension encompassed 49.5% of the total variation of the data. This dimension is strongly positively correlated to levels of clay (r2 = 0.88; p < 0.01), rate of slit (r2 = 0.83; p < 0.01); and strongly negatively correlated to the rate of sand (r2 = −0.997; p < 0.01). The second dimension explain 26.1% of the total data variation. Altitude and AMF spore density with r-square value of 0.81 and 0.79 respectively (p < 0.01) have a positive effect on the second dimension. Along this dimension localities with low altitude (Mbanga and Penja) were separated from the localities of high altitude where high rates of AMF spore density were observed like Bassamba, Tonga and Melong.
According to the dataset, the PCA analysis based on the physicochemical properties; altitude; AMF roots colonization (spore density and mycorrhizal frequency) revealed that the first two dimensions express 55.4% of the dataset inertia (Figure 9(a)) also suggested to interpret the dimensions greater or equal to the third one. Then, if the third and the fourth dimensions are included in the interpretation, the total information contained in the PCA will therefore be 75.1% (Figure 9(b)). The first dimension encompassed 41.7% of the total inertia and is significantly (p < 0.001) positively correlated with soil sand content, nitrogen, CEC; and negatively correlated with pH, soil slit and clay content. The second dimension is significantly positively correlated with C/N, organic matter and organic carbon; and negatively correlated with available phosphorus. The mycorrhizal frequency (r2 = 0.61; p < 0.01) and the available phosphorous (r2 = 0.54; p < 0.01) positively affected the third dimension while the spore density after trapping (r2 = 0.42; p < 0.05) and altitude (r2 = 0.75; p < 0.01) negatively correlated with the said dimension. Taking into account the third dimension, samples from Bassamba 2, Binguela 3, Bokito and Penja 2 characterized with high rate of frequency of mycorrhizal colonization and are opposed along the same dimension to samples from Bassamba 3, Bafang 2 & 3 and Ntui 3 characterized by low spore density after trapping.
4. Discussion
4.1. Soil Physico-Chemical Properties and Cacao Preference
Multi-environment experiments are a primary focus in selecting AMF able to help plants to develop adaptation strategies to soil disturbances, pollution, drought and salinity. This study described soil physico-chemical properties and provides rates of AMF distribution in contrasting cacao environment cultures in Cameroon. Soil analysis according to the FAO texture triangle showed that, four textural classes were observed in the study sites: sandy-loam, sandy-clay-loam; loam sandy and clay loam. Soils with a coarse texture, such as sandy-loam, loam-sandy texture offering good root penetration are light and filtering; but because of their richness in sand, they are sensitive to leaching [35] [36] [37] . The cacao tree establishes itself better on soils with a sandy-clay texture [38] [39] or on clay and clay-loamy soils [40] . However, soils with sandy loam and loamy-sandy texture
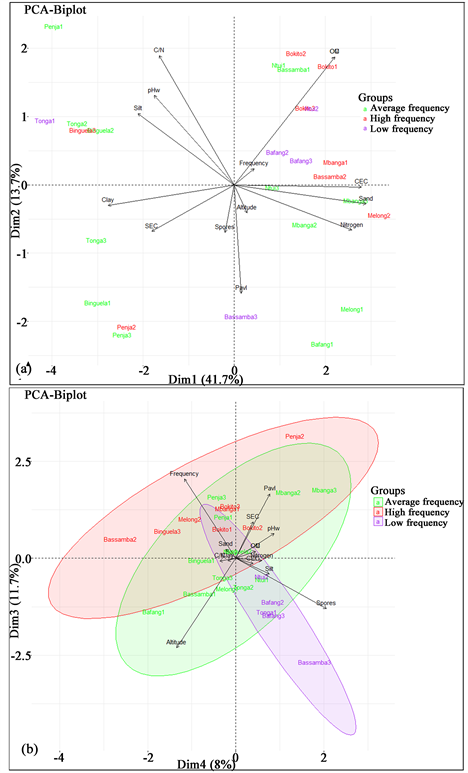
Samples were color-coded according to frequency of mycorrhizal. Soil sand contain (Sand); soil clay contain (Clay); soil slit contain (Slit); Carbon/Nitrogen ratio (C/N); pH water (pHw); Sum of Exchangeable Cations (SEC); Organic Matter (OM), Organic Carbon (OC); Cation Exchange Capacity (CEC); Soil organic Nitrogen (Nitrogen); AMF Spores density after trapping (Spores).
Figure 9. PCA analysis with soil physicochemical parameters, altitude, AMF spore density after trapping and mycorrhizal frequency.
are acceptable in cacao-growing, especially if they are rich in organic matter. That increase in organic matter can be achieved through a combination of practices, such as using manures, compost, tillage reduction, mulching [41] .
Soil quality is a holistic term used to describe many soil functions such as soil health, crop productivity, and nutrient content [42] . Soil pH influences the solubility and availability of nutrients, bacterial and fungal composition, and diversity [43] . Organic carbon content is essential for bacterial and fungal cellular metabolism, survival and growth [44] . The current study demonstrates that soils from the different locations were acidic except soil from Tonga which was neutral. Cacao develops well on soils with a pH of 5.1 - 7.0 [45] . Humid tropical soils are known to be acidic, as they are subject to frequent base leaching [46] . Nitrogen content of sampled soils was between 0.13% and 0.45%, which are suitable for cacao, as they are above the necessary critical nitrogen threshold of 0.09% [47] [48] or 0.12% [49] . High level of total nitrogen could be the consequence of mineralization of organic matter at the litter [50] and by the ability of certain shade plants, such as shrubby legumes found in cacao farming, which fix and concentrate atmospheric nitrogen in the soil. The average level of organic carbon and organic matter observed in the different locations met the average requirements for cacao growth and development [45] . High level of organic matter impacted positively the CEC of a given soil. This correlation has been demonstrated. However, in all the locations investigated, soil C/N was below the threshold (C/N = 12) necessary for optimal growth and development of cacao tree, except for Tonga and Penja area where the C/N ratio was 16.16 and 13.34 respectively. Such lower ratio is attributed to the high average soil temperatures and the intense microbial activity. The C/N ratio could be increase by using more organic manure or straw to increase the level of organic carbon [51] . High C/N ratio indicates a slower decomposition of organic matter while reverse trend expresses high nitrogen content as a result of soil humification. The CEC of a soil is the maximum quantity of cations that the soil can retain. The high values of the CEC in the study areas could be attributed to the high levels of organic matter as recorded during the analyses. Our field data in terms of Phosphorus content indicate that agroclimatic conditions impact their availability. Thus, soils from the monomodal agroecological zones (Mbanga, Melong and Penja) locations have more available P than Ntui and Bokito counterparts. Tropical soils are known to be low in phosphorus [52] , the high rates observed in some areas could be the result of farmer amendments with fertilizers as reported during sampling.
4.2. Relationship between Environmental Variable and AMF Root Colonization
Based on the current data, soil physico-chemical properties and altitude had a significant effect on AMF spore density and root colonization ability. It was observed that localities like Bassamba and Tonga in the Western highlands and, Bokito and Ntui in the bimodal zone, with high AMF spore density before trapping are very poor in available phosphorus. That was illustrated by the PCA analysis which showed a negative correlation between AMF spore density and available phosphorus. AMF play a major role in the mobilization of nutrients, especially phosphorus. They are more abundant in soils low in available phosphorus. Indeed, plant nutrition is limited by low phosphorous availability due to inability to uptake phosphorous in orthophosphate ions form directly through roots. Many authors reported that high amounts of available phosphorus reduce AMF spore density and diversity in soil [53] [54] [55] [56] [57] . This can be explained by the fact that due to a lack of available phosphorous, the plant initiates a molecular dialogue with AMF that reprograms both host and symbiont to commit to symbiosis, leading to contact and plant accommodation of arbuscular mycorrhizal fungi intraradical hyphae inside the roots and to intense exchange between the two partners. Symbiosis therefore enables the fungus to complete its life cycle by producing spores to reinfect future hosts [58] . However, some authors report that an increase of available phosphorous is positively correlated with AMF spore density that can be explained by a change in pattern of resource allocation between the hyphal networks as functional structures which provide nutrients to host plants and the spores as survival resting structures [59] [60] . Samples from Melong situated in a monomodal zone effectively displayed high AMF spore density but a high rate of available phosphorus but this could more likely be linked to soil pH.
Concerning the pH, it was observed that localities that displayed high AMF spore density like Tonga, Ntui and Bokito also distinguished themselves with pH (6.33 - 6.67) values close to neutral. It was reported by [61] that AMF had a preference for neutral and alkaline soil pH. In contrary to the previous observation, samples form Melong had a high AMF spore density but a soil pH close to high acid. This locality also displayed a high rate of available phosphorous known as the key factor in AMF distribution [55] . Thus, a high AMF sporulation in acidic milieu can be considered as resistant behaviour to help survive adverse environmental conditions.
For altitude variation, high AMF spore density strongly correlated with altitude. Some authors reported that AMF spore density and diversity increase with altitude elevation [62] , while others observed variations linked to environmental conditions specific to the study site [63] . Our results showed that the samples collected at high altitude are very low in available phosphorus. Shara et al. (2021) [64] demonstrated a decrease in the availability of P, Ca and Mg to plants due to increased soil leaching. A high abundance of spores at high altitudes would therefore be an adaptation of the plants that initiate symbioses in order to adapt to global conditions.
5. Conclusion
For the first time we provide a base to predict arbuscular mycorrhizal fungi distribution associated to cacao rhizosphere in Cameroon. The results obtained herein revealed that the soil from the western highlands zone, monomodal zone and bimodal zone are adapted to cacao nutrition preference. Our findings have also highlighted that soil properties, available phosphorus and pH and altitude were all strongly correlated with AMF root colonization ability. Further work will focus on the molecular identification of the native AMF in the cacao rhizosphere to bring out the diversity of arbuscular mycorrhizal fungi and their variation according to different agroecological zones of Cameroon.
Acknowledgements
We express our gratitude to all the farmers in accessing these plantations. We also want to extend our gratitude to Mr Kouhou Djaouro from the Ministry of Environment, Protection of Nature and Sustainable Development who contributed to the setting up of the sampling area map. The donation of laboratory equipment by the Alexander Von Humboldt Foundation and DAAD to Nicolas Niemenak is appreciated.