Acute Chest Syndrome and Pulmonary Embolism in Sickle Cell Disease Case Report: About one Case Admitted at the Mixed Medecine and Sickle Cell Anemia Center (M.M.SC.A) Kinshasa in the Democratic Republic of the Congo ()
Keywords:

1. Introduction
Sickle cell disease (SCD) is an inherited hematological disorder. Normal hemoglobin consists of 2 alpha and 2 beta units, referred to as hemoglobin A. In individuals with sickle cell anemia, inheriting abnormal genes from both parents results in defective beta unit synthesis, leading to the formation of hemoglobin S. Hemoglobin S is predisposed to sickling under specific conditions [1] [2] . This sickling phenomenon arises from a mutation in beta-globin, where valine replaces glutamic acid. Globally, approximately 50 million people are affected by SCD, with a prevalence pattern similar to that of malaria, particularly among citizens of Middle Eastern, sub-Saharan African, and other Mediterranean countries [1] [2] .
Patients with sickle cell anemia experience various complications, notably recurrent hemolysis and vaso-occlusive crises, along with the risks of stroke and deafness, and lung damage represents nearly 20% to 30% of the causes of mortality [3] [4] [5] .
Acute chest syndrome (ACS) is denoted as an acute pulmonary illness in individuals with sickle cell disease. ACS is defined by the presence of new pulmonary infiltrates on chest radiography, accompanied by symptoms such as fever and chest pain. Cardinal signs of pulmonary diseases, including tachypnea, cough, and dyspnea, are also present. Vaso-occlusion within the pulmonary vasculature is believed to be the cause of ACS, leading to deoxygenation of hemoglobin, sickling of erythrocytes, and subsequent occlusion, resulting in ischemia and injury to the vascular endothelium. ACS progresses rapidly and stands as a leading cause of mortality in individuals with sickle cell disease [3] .
Pulmonary embolism (PE) is a life-threatening and potentially lethal condition. Its incidence in children with sickle cell disease is likely underestimated, with rare pediatric case reports in the literature. Moreover, symptoms of pulmonary embolism can mimic those of acute chest syndrome [6] .
2. Objectives
Demonstrates that pulmonary embolism should be considered in the differential diagnosis for any sickle cell patient presenting with an insidious onset of chest or thorax pain, especially in those with other predisposing factors for venous thrombosis.
3. Observation: Clinical Case
A 22-year-old patient weighing 52 kg sought emergency care for dyspnea, multifocal pain, dry mouth, and physical asthenia. The patient has a history of sickle cell disease since the age of 5, regularly monitored at the Mixed Medicine and sickle cell anemia center and the MONKOLE Center Hospital. He has been on Hydrea® for about 4 years and folic acid since childhood, experiencing priapism as a significant complication. The patient’s specific vaccination schedule is not up to date.
Anamnesis revealed multifocal osteo-joint pains, headache, and a productive cough for 2 weeks without fever. Treated initially for vaso-occlusive crisis with diclofenac 75 mg and paracetamol 1 g infusion without success, the patient was then brought to the Mixed Medicine and Sickle Cell Anemia Center (commonly called Mabanga Hospital) for further evaluation.
Upon physical examination, the patient exhibited altered general condition due to pain, with an Analog Visual Scale (AVS) rated at 6/10, and moderately colored palpebral conjunctiva. The patient showed signs of respiratory distress, including polypnea at 39 cycles per minute, discreet intercostal retraction, and nasal flaring. Oxygen saturation ranged between 88% and 90% in ambient air and 95% under oxygen therapy at a nasal cannula with a 4-liter flow per minute. The patient presented regular tachycardia at 120 beats per minute, with blood pressure at 110/60mmHg. Examination of the digestive, spleen, and lymph node tract was normal. The pain was noted at the long bones.
Given the presentation of generalized osteo-joint pain, cough, dyspnea with decreased air entry, and rales in the pulmonary auscultation, a suspected acute chest syndrome (associated with bronchopneumonia) was considered. The patient initially received oxygen therapy, multimodal analgesia, and hyperhydration with isotonic saline solution enriched with electrolytes.
On the biological level, critical findings included anemia at 7.6 g/dl, hyperleukocytosis at 20,000/mm3 with lymphocytosis predominance, and normal urea and creatinine. A chest X-ray revealed an image suggesting bilateral interstitial pneumopathy. Considering these results, bi-antibiotic therapy with beta-lactam (amoxicillin and clavulanic acid) and a macrolide (azithromycin) was added.
Radiographic images did not justify the severity of the clinical presentation. Suspecting pulmonary embolism, the PERC rule yielded a score of 2/8, the calculated WELLS score was 5.5 (moderate clinical probability) with a 19% chance of pulmonary embolism, and the Geneva score was 7 (intermediate probability) with a 28% possibility. D-Dimer levels were higher than 10,000 ng/ml (normal range: 500 ng/ml), and the electrocardiogram (ECG) was normal. Blood gasometry could not be performed.
The combination of pulmonary embolism with acute thoracic syndrome led to the addition of low-molecular-weight heparin (enoxaparin) with INR control and prothrombin control every 3 days. The cardiac echography was normal, and a blood transfusion was administered 2 days after admission due to an Hb level of 6 g/dl. Adherence to treatment was favorable, with respiratory distress improving and discontinuation of oxygen therapy. After 7 days of injectable enoxaparin treatment, the patient was switched to Rivaroxaban Accord (XARELTO®) tablet 20 mg/day for 6 months. The patient was discharged after 9 days of hospitalization.
After 5 weeks of treatment, a chest angioscanner confirmed bilateral pulmonary emboli distal to the right. A follow-up D-Dimer control after 6 months of treatment showed a clear regression, with a value of 3400 ng/dl (normal range: <500 ng/dl).
4. Discussion
Acute chest syndrome (ACS) is defined by the occurrence of chest pain, dyspnea, and cough, accompanied by fever and the appearance of a clinical and radiological pulmonary abnormality. It is a feared complication in sickle cell disease, representing the leading cause of mortality and a source of recurrence and sequelae (hypoxia favoring painful crises). Understanding the physiopathology of ACS is crucial for effective treatment [7] [8] .
・ The physiopathology is complex, involving several potential causes:
・ Thrombosis in situ facilitating pulmonary microcirculation.
・ Pulmonary infection (pneumococcal, hemophilus, mycoplasma), which appears to predominate in children.
・ Secondary atelectasis in bone infarction, particularly in the costals.
・ Bowling emboli at the bottom-starting point (confirmed by bronchoalveolar lavage with a special staining technique).
・ Intravascular pulmonary sequestration of sickled erythrocytes leads to lung injury and infarction [9] .
With our patient, the clinic was marked mainly by multifocal osteoarticular pain, and a morbid coughing entity was found in the association as a cause of the acute thoracic syndrome disease. The limited extent of the radiological lesions was not favorable for our diagnosis, but, on the other hand, the thoracic angioscanner confirmed the pulmonary embolism 5 weeks later.
As the clinical signs and functional symptomatology of PE are not sensitive and not very specific, it is appropriate to take into account the pre-test clinical probability, which corresponds to the observed prevalence of PE in case of suspicion of this diagnostic. This a priori evaluation of clinical probability remains an essential prerequisite for the diagnostic strategy and can be empirical or based on the well-defined prediction rules of WELLS or GENEVA [10] [11] .
All six homozygous sickle cell disease patients in a retrospective study (conducted in Tunisia) collected over a three-year period, divided into four men and two women with an average age of 25 years, were hospitalized for abrupt chest pains associated with dyspnea. Two patients had a fever [12] .
The chest X-ray (Figure 1) revealed parenchymal consolidations in all patients, peripheral segmental (n = 4), and lobar (n = 3). Thoracic angioscanner
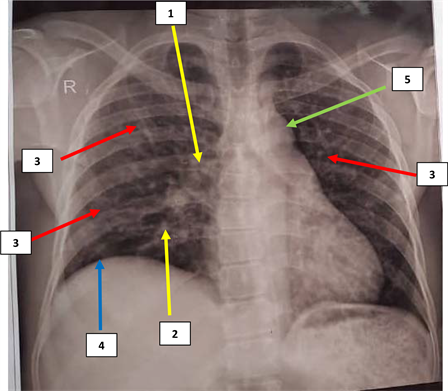
1. Hilum of the lung increased in size, 2. appearing to end in a mouse tail. 3. Highlighting nodular opacities distributed diffusely in both hemithoraces; 4. Ascent of the right diaphragm. 5. Unwinding of the aorta. Source: Medical Folder of our patient.
Figure 1. Frontal chest X-ray of our patient.
highlighted arterial thrombosis in four patients, lobes (n = 2), segmentaries (n = 3), or sub-segmentaries (n = 2). Pleuro-parenchymal study shows triangular consolidations under triangular bases (n = 4), mobile glass hyperdensity beaches (n = 1), extended parenchymal consolidation firms (n = 3), and pleural effusion (n = 4) [11] .
Bronchoalveolar washing (BAW) highlighted the presence of spumous cells, indicating fatty embolism in one patient [11] .
Cardiac Doppler echography, conducted to investigate pulmonary arterial hypertension (PAH), was normal. In a study in France, covering 29 patients with acute chest syndrome (ACS) due to sickle cell disease, where 22 underwent transthoracic echography (TTE), the presence of severe PAH was associated with a more clinical presentation and a more severe evolution (pulmonary embolism and multi-organ failure leading to death) [13] .
Our patient’s hemogram showed anemia at 7.6g/dL, hyperleukocytosis at 20,000/mm3 with a predominance of polynuclear lymphocytes during the acute chest syndrome (ACS), as in vaso-occlusive crises. The C-reactive protein increase is almost constant and indicates bacterial infection [14] .
Treatment is based on a tripod: hydration, oxygenation, and analgesia covering an encrypted and atypical bacterium. In severe forms, the sole specific treatment is transfusion or exchange transfusion based on hemoglobin levels. Preventive transfusion or exchange transfusion may be considered in at-risk patients, especially in particular circumstances (pregnancy, surgical procedures). This decision is made case by case based on transfusion history [14] [15] .
In the context of prevention, incentive spirometry (respiratory physiotherapy) should be prescribed for hospitalized sickle cell disease patients, especially during vaso-occlusive crises [6] [14] .
Our patient also benefited from this treatment regimen (hydration, oxygenation, and analgesia associated with antibiotic therapy). Molecular low-weight heparin was added once the diagnosis of pulmonary embolism was suspected (based on clinical and radiological signs and elevated D-Dimer levels). Transitioning off oxygen therapy was done gradually. Although incentive spirometry is not a common practice in our health structure, it was not carried out. A punctual and manual transfusion exchange was indicated but not performed due to the high financial cost it required.
5. Conclusions
Patients with sickle cell disease exhibit a baseline hypercoagulable state and are at an increased risk for venous thrombosis and pulmonary embolism [16] . Our case report emphasizes the importance of a high index of suspicion in sickle cell patients presenting with pleuritic chest pain.
Angioscanning allows for the identification of the etiology of acute chest syndrome in sickle cell disease by revealing the presence of arterial thrombosis. In other cases, it aids in considering alternative etiologies (infection, fatty embolism, etc.) based on clinical and biological data.
The combination of pneumonia with pulmonary embolism in acute chest syndrome must be suspected in the presence of discordance between clinical and radiological signs. D-Dimer evaluation for treatment assessment is underway.
Consent
Oral and written patient consent and that of their guardian were obtained before drafting this article.
Financing
No funding was obtained for the drafting of this article.
Contribution
All authors contributed to the writing of this case report.