The Risk Factors of Lymphedema in Breast Cancer Patients Post Axillary Clearance ()
1. Introduction
Breast cancer is the most common cancer affecting females in Saudi Arabia with a prevalence of 21.8% of all cancers, high incidence of two thousand cases per year, and mortality rate of 18.7% [1] [2] [3] . There are many factors that may contribute to developing breast cancer such as, gender in which female-to-male ratio is 100:1 to 150:1. Also, the risk increases with age and family history. Genetic mutations account for 10% of all breast cancers [4] . Treatment options vary from surgery, chemotherapy, hormonal therapy, and radiotherapy. When surgery and radiation are the treatment of choice, they are usually accompanied by sentinel lymph node biopsy and/or axillary lymph node dissection [5] . Sentinel lymph node is the first lymph node that harbors local metastasis, it receives direct drainage from the primary tumor thus it reflects the extension of the primary cancer [6] . In the presence of only one or two sentinel lymph nodes containing evidence of cancer, axillary lymph node dissection may not be necessary. However, if three or more sentinel lymph nodes are found to be positive, then axillary clearance is most likely recommended [5] . If no radioactive nodes are found, then a formal ALND is required. ALND also takes place in the operating room, where all level I and II nodes are resected, and it is considered adequate if more than 10 nodes are collected [4] . There are many complications to ALND including: Axillary vein thrombosis which is a sudden early postoperative swelling, and it is rare; Lymphedema, a slow developing swelling of upper extremity or breast or chest, over a period of 18 months. Other complications include Lymphangiosarcoma, Stewart Treves syndrome, and intercostal brachiocutaneous nerve injury [4] . Most breast cancer patients might have clinically negative lymph node involvement, but sentinel lymph node biopsy can reveal otherwise. Having a positive lymph node involvement and the number of lymph nodes involved are the two most important factors for the additional treatment options for the axilla [6] . The two management modalities are axillary Lymph nodes clearance or axillary radiotherapy. The choice of treatment must be tailored to the individual factors including risk of complications and extension of lymph node involvement [6] . Breast Cancer Related Lymphedema (BCRL) is an undesirable chronic complication of aggressive treatment of breast cancer when the act of axillary lymph node dissection (ALND) or radiotherapy is applied [7] [8] [9] . It is a serious complication of axillary clearance and characterized by accumulations of protein-rich fluid in soft tissues that interrupt the lymphatic flow [7] [10] . Quality of life for BCRL patients is going to be affected and impaired because BCRL has poor functional recovery. Survived patients are prone to have physical and psychological impairments when they face disabilities in their lives [11] . Several factors are attributable to lymphedema including mastectomy combined with ALND, local irradiation of lymph nodes and increasing age or body mass index (BMI) are all linked to increase the rate of BCRL [8] [12] . This cross-sectional single center study aims to identify the prevalence rate of lymphedema and other complications in breast cancer patients who underwent axillary clearance from 2016 to 2019 in King Abdulaziz Medical City (KAMC)—National Guard Hospital (NGH) in Jeddah, Western region of Saudi Arabia. Additionally, compare the rate of complications in routine axillary clearance with sentinel lymph node biopsy vs. sentinel lymph node biopsy-oriented management with radiation.
2. Methodology
This is a cross sectional single center study, conducted at King Khalid National Guard Hospital in King Abdulaziz Medical City (KAMC) in Jeddah, Saudi Arabia. Also, in collaboration with King Saud bin Abdulaziz University for Health Sciences (KSAU-HS) and King Abdullah International Medical Research Center (KAIMRC). KAMC is a complex medical city and a tertiary referral center, famous for receiving breast cancer cases from the western region. Data were collected after reviewing medical record files of all patients that fit the criteria from 2016-2019. Most subjects of this study are National Guard dependent, others are referred cases. Inclusion and exclusion criteria were the following: we included only female breast cancer patients, age range was 35 - 60 years, who underwent, SLNB, ALND, lumpectomies, wide local excisions, and mastectomies. We excluded incomplete follow ups, and male patients. Originally, the sample size was calculated with a 95 percent confidence level, margin of error of ±5%, an estimated population size of 520 and prevalence of 50%. The required minimum sample size was determined to be 222. As the sample size is small, we included all patients that fulfill the criteria during the study period from 2016 to 2019 following a consecutive sampling technique. We collected data from the electronic medical records using electronic data gathering sheets. We gathered the following information: patient age, family history, body mass index (BMI), lymphedema, other complications, type of surgery, chemotherapy, and radiotherapy. Data was organized on Microsoft excel spreadsheets.
3. Statistical Analysis
Data were checked for completeness and the errors were corrected. Categorical variables were presented as frequencies and percentages. Continuous variables were presented as mean and standard deviation. Prevalence of complications was presented. The prevalence of complications was compared by Chi-Square test. The analysis was performed with 95% confidence interval using the Statistical Package for Social Science (SPSS), version 23.0 (IBM, Armonk, NY, USA).
4. Results
A total of 253 breast cancer cases were included in this study, with a median age of 53 years (Table 1 & Table 2), 52.7% were postmenopausal and positive family history was present among 21% of cases. Further, 90.9% of the cases had unilateral disease and staging was as follows: stage I 14.5%, stage II 45.2%, stage III
![]()
Table 1. Distribution of all cases by age, age at diagnosis, and BMI (n = 253).
![]()
Table 2. Patients’ characteristics.
37.1%, and stage IV 3.2%. As per Chart 1, Mastectomy was done in 73.4% cases and lumpectomy was performed in 34.1% of cases. For local control of the tumor, 93.3% of patients had SLNB and 49% were positive for which some underwent ALND, or Radiotherapy. Axillary dissection was performed in 69.6% of our patients (either after positive SLNB or had positive lymph nodes clinically). Radiotherapy and chemotherapy were given to 71.8% and 80.4% of cases respectively. Among the chemotherapy (chemo) recipients, 40.2% received adjuvant chemo, 54.5% received neoadjuvant chemo, and the remaining 5.3% received both. As per the number of cycles, most of the cases received an average of 3 to 6 chemo and/or radiotherapy cycles. The median radiotherapy course duration was four months. In this study, we are reporting complications post surgery but in depth about lymphedema. The most prevalent complication was pain 42.1%, and the least prevalent was cellulitis 4%. Also, seroma developed in 18.3% cases, paresthesia noted in 5.6% of cases, winged scapula was reported as 2%, weakness and necrosis were seen in 6% and 13.1% of cases respectively (Chart 2). Axillary
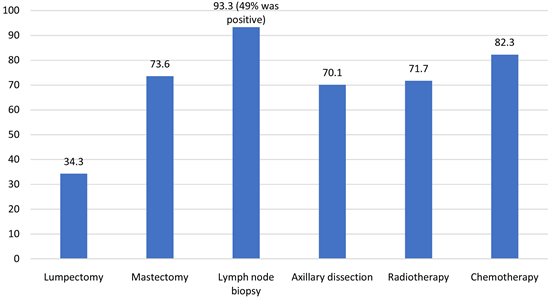
Chart 1. Procedures underwent (%).
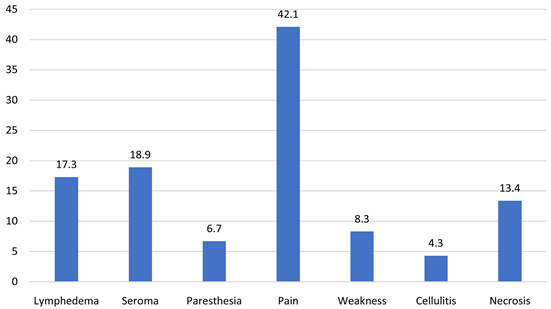
Chart 2. Prevalence of complications (%).
vein thrombosis and lymphangiosarcoma were reported in none of the patients (0%). The prevalence of complications was not statistically significantly different between those who had mastectomy or those who hadn’t (all p-value > 0.050). Lymphoedema was seen in 16.1% of the cases, 84.4% of the patients that developed lymphedema also underwent ALND, and 12.9% and 14.4% received radiotherapy and chemotherapy respectively. Prevalence of lymphedema was significantly lower among patients undergoing lumpectomy (10.3%) compared to others p-value = 0.034 (Table 3 & Table 4). Lymphedema was observed in breast cancer stages as follows stage I 1.2%, stage II 7.2%, and stage III 5.2%, stage IV 0%. Patients with body mass index (BMI) of <25 kg/m2 reported a prevalence of lymphedema of 2.4% , BMI of >25 - 29 kg/m2 was 4.8%, >30 - 39 kg/m2 was 7.2%, and BMI of >40 kg/m2 was 1.6%. The death percentage was highest among mastectomy patients (10.2%). For lumpectomy patients’ death percentage was considerably lower (4.6%). Overall, 8.3% fatality was observed (Chart 3).
![]()
Table 3. Relationship between mastectomy and complications and survival.
![]()
Table 4. Relationship between lumpectomy and complications and survival.
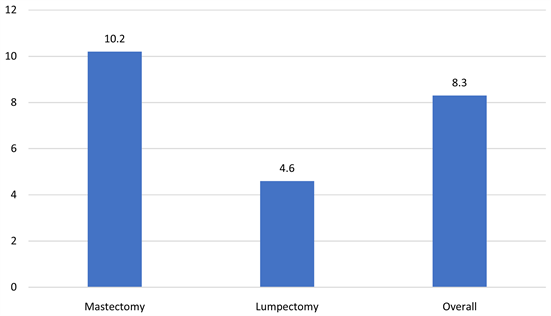
Chart 3. Death (%).
5. Discussion
Through collecting data from electronic medical files, we were faced with challenges in our sample size, as some patients did not fit our criteria due to age limit (more than sixty years of age) or advanced breast cancer (stage IV). So, in order to increase our sample size, we included few stage IV breast cancers and extended our search to include cases up to December of 2019 unlike the original plan of searching patients up to august of 2019. We were also challenged by some complicated cases, we had a case of bilateral breast cancer with different stages, for example, the patient had left breast cancer stage II and right breast cancer stage IV which was enrolled in our study. As for the reporting complications, the intercostobrachial nerve injury was not reported in the results due to non-preserving dissection technique used in almost all cases. Also, there was an overlap between the percentages of lumpectomy and mastectomy cases, and this is due to few cases of patients having undergone both lumpectomy and mastectomy in the follow up period. The study results indicate that there is no significant difference in prevalence of overall complications in the different treatment modalities. In similar studies, Preetinder Brar et al., an Indian paper published in 2011, where they compared two groups of breast cancer patients (Mastectomy vs. Lumpectomy) and observed for complications after axillary clearance, had similar reporting complications where pain and numbness were the most common, and found no statistically significant difference in the reporting of symptoms in the two groups [12] . In an Australian paper, they compared sentinel node biopsy-based management and node biopsy with routine axillary clearance while measuring the outcomes of lymphedema for 5 years after the intervention. The study found that sentinel node biopsy-based management had lower risk of morbidity regarding patients concerns and limb symptoms [13] . This emphasizes that having less invasive procedures result in less complications, similarly the effect of breast conserving surgery BCS like lumpectomy is less invasive than mastectomy. As in our study, we reported lower prevalence of lymphedema in the lumpectomy group compared to the mastectomy group who underwent ALND. Another study, Melam et al. (2016) revealed that surviving patients have improved and a good quality of life when these women had a certain strategy such as Complete Decongestive Therapy (CDT) after surgery. Moreover, manual lymphatic drainage, compression garment worn for long hours per day, remedial exercises and a home program have improved quality of life for such patients [11] . Thus, we suggest and encourage patient education about the complications and their prevention during hospital stay regardless of the chosen procedure. According to Toan T, et al. (2017) the incidence of lymphedema ranges from 3% - 65%, and it depends on the treatment, mode of lymphedema diagnosis, and the length of follow-up. In the study, they followed approximately 1700 patients looking for Breast Cancer Related Lymphedema (BCRL) for years after surgical intervention and concluded that no BCRL occurred among the group with no axillary surgery, and that BCRL is a consequence after breast cancer management and the risks multifactorial but included chemotherapy, radiation, ALND, more advanced disease stage, and higher body mass index [10] . As in our paper, patients with higher BMI and advanced stage reported higher prevalence of lymphedema. In conclusion, many studies report similar results.
Acknowledgements
The completion of this study could not have been possible without the help of Sondos Rawas, Shatha Almasri, Muhanned Hatem, Rana Alghamdi, and Leen Othman. We thank them for their efforts in the data collection.