1. Introduction
In recent decades, fisheries resources have come under considerable pressure (overfishing, climate change, etc.), leading to a considerable decline in the potential of fish stocks in estuaries, rivers and seas [1] . To deal with this over-exploitation of fisheries resources and to make up for the shortfall in capture fisheries, the Senegalese government committed itself to developing aquaculture with the creation in 2006 of the National Agency of Aquaculture (NAA), which is responsible for implementing Senegal’s aquaculture policy. To further illustrate the commitment of the Senegalese government, aquaculture has been included among the 6 priority sectors and 27 flagship projects that will create jobs and wealth and boost the country’s growth. Alongside this, institutions were created such as the Directorate of Inland Fisheries (DPC), the National Agency for Agricultural Integration and Development (NAAID) and the Community Agricultural Areas Programme (PRODAC). Among the species farmed in Senegal, tilapia is the group of fish whose farming has seen the strongest growth over the last ten years [2] . However, the development of this production is faced with a problem linked to the high reproduction of the species and the difference in growth between males and females in favor of the males. With this in mind, a technology transfer project financed by the National Agricultural Research Fund (NARF) and led by CRODT between 2016 and 2017 worked with development structures such as NAA and NAAID to solve the problem of the availability in quantity of male tilapia fry by using the hormone methyl testosterone. However, the high cost and difficulties in importing the synthetic hormone currently limit its use for the production of male monosex tilapia in developing countries.
With this in mind, it is important to find methods of producing male monosex fry using Garcinia kola powder as a feed supplement which, with its bioactive principles, influences various parameters including appetite stimulation and fish growth in aquaculture [3] [4] . It is widely available and distributed throughout the country. It is a medicinal plant commonly used in traditional medicine, rich in flavonoids, saponins, steroids, tannins and terpenoids [5] [6] . They also act as endocrine modulators and have anti-estrogenic properties [7] . In this way, they can block the synthesis of estrogens through their ability to inhibit aromatase and reduce the level of estrogens in the body. Inhibiting aromatase enzyme activity or blocking estrogen signaling pathways leads to functional masculinization [1] . The objective of the present study is to contribute to the improvement of the piscicultural productivity of O. nilotica. The effect of Garcinia kola on the mass production of monosex male fry of O. niloticus was evaluated, and the optimum dose and lethal dose of Garcinia kola on sexual inversion, survival and growth performance were determined.
2. Materials and Methods
Study area
Located 15 km from the department of Kedougou, the Community Agriculture Area fish farm is in the commune of Bandafassi, in the village of Itato. It is close to the River Gambia, a strategic choice for supplying water to the ponds. The following infrastructure is on site: a pumping station with a 10,000 m3 water reservoir; a hatchery with a capacity of 100,000 fry/month; grow-out units consisting of 80 concrete tanks, including 10 of 10 m3 tanks, 20 of 20 m3 tanks and 50 of 50 m3 tanks; a feed and equipment storage warehouse.
This study was carried out at this fish farm between March 2022 and May 2023. Picture 1 shows the fish farm on the community agriculture area.
2.1. Material
To carry out this study, we used as biological material the species O. niloticus, a strain from the Senegal River valley (NAA Richard TOLL). The experiments began with the recovery of larvae (average weight between 0.01 and 0.02 g) from broodstock (body mass between 100 and 200 g) well adapted to life in captivity at the agriculture community estate of fish farm. These larvae were subdivided into 12 batches (12 aboveground tanks of 1 m3 each) of 150 individuals each +−5%. We used two (02) 10 m3 concrete tanks for breeding, with a ratio of 1 male to 3 females. For the incubation equipment, we used: six (06) bottles of zoug for incubating the eggs; six (06) trays for resorbing the yolk sac; six (06) happas for recovering the larvae and one (01) 12 kV submersible pump for supplying water to the incubation table. To formulate the hormone feed, we used 60 mg/kg of 17-alpha-methyl-testosterone powder; 95% alcohol (Ethanol); very fine industrial feed containing 40% protein and glassware (test tubes, spoons, trays, bottles; gloves, gown, boots, bags, cloth, mask). For the formulation of the feed containing the Garcinia kola powder: Garcinia kola (bitter kola) from the Republic of Guinea; 95% alcohol (ethanol) and very fine industrial feed containing

Picture 1. Itato DAC fish farm (Sarr, 2022).
40% protein. And equipment to measure physical and chemical parameters (thermometer: to measure the temperature of the water, oximeter (DO9100): to measure the dissolved oxygen in the water, pH-meter: to measure the hydrogen potential of the water, GPS: to locate the study area, scales: to measure weights).
2.2. Methods
2.2.1. Broodstock Reproduction
To obtain twelve (12) batches of fry, Tilapia broodstock were spawned in two concrete tanks of 10 m3 each, with a sex ratio of 1 male to 3 females in the tank. The water temperature varied between 25 and 30˚C. These spawners are fed three times a day (which varies according to their needs and behavior. Parameters, such as temperature, are checked three times a day (9 am, 1 pm and 5 pm) and oxygen twice a day (10 am and 4 pm). The eggs incubated by the females are recovered.
2.2.2. Recovery of Eggs and Larvae
During this study, broodstock were maintained under optimal abiotic rearing conditions. Several signs allow the identification of incubating females, such as the appearance of a dark band on the forehead and black spots on the flanks, a rapid and discontinuous clouding with fairly aggressive behavior towards other individuals present in the tank [8] . Eggs were collected by capturing the females one by one in each pond using a small-mesh net. Picture 2 shows the egg and larva recovery phase.
2.2.3. Egg Incubation
The incubation phase was carried out artificially. Two thousand seven hundred and thirty (2730) already fertilized eggs were collected and incubated in zoug bottles (1.5l). The incubation temperature during the experiment was 28˚C until the resorption of the yolk vesicle between days 5 and 7. The larvae obtained were then weighed, counted and divided into 12 batches of 150 individuals each. Picture 3 shows the incubation phase.

Picture 2. Egg and larva recovery phase (Sarr, 2022).
2.2.4. Feed Preparation
The feed used during the experiment was industrial “GOUESSANT AQUACULTURE” in 20 kg bags. Picture 4 shows the images A and B.
In this experiment, 12 diets, each corresponding to a treatment, were developed, with 4 controls (2 negative and 2 positive) and 8 tests (Garcinia kola).
· The negative controls were applied directly (simple food containing 40% protein);
· The positive controls were obtained by dissolving 60 mg of hormone 17 alpha-methyl testosterone in 250 ml of 95% ethanol in an industrial foodstuff containing 40% protein. To evaporate the alcohol, the feed was carefully mixed and dried at room temperature in the dark. The biochemical composition of the feed is given in Table 1.


Picture 3. Zug bottle incubation phase (Sarr, 2022).
 (a)
(a)  (b)
(b)
Picture 4. (a) Bag of industrial feed with 40% protein “GOUESSANT AQUACULTURE”; (b) Industrial feed treated with the hormone 17 alpha-methyl testosterone in 250 ml 95% ethanol.
· Concerning the preparation of the test foods, 8 diets were prepared, with 2 diets for each dose of G. kola (Diagram 1). To this end, fresh G. kola seeds were cleaned with distilled water, cut into small pieces, dried separately at room temperature for seven days, made into powder, and then passed through a 0.1 mm mesh sieve [8] . For each kilogram of food, G. kola samples of 10, 20, 30 and 40 g were macerated respectively in 50, 100, 150 and 200 ml of 95% ethanol in the dark at room temperature for 24 h.
Four times four (4 × 4) test diets: B5 and B6 (10 g of G. kola powder each), B7 and B8 (20 g of G. kola powder each), B9 and B10 (30 g of G. kola powder each) and B11 and B12 (40 g of G. kola powder each) for one kilogram of food containing 40% protein. Diagram 1 shows the experimental set-up adopted.
![]()
Table 1. Physical and chemical parameters of the different rearing systems.

Diagram 1. Experimental scheme adopted.
2.2.5. Feed Ration and Feeding Frequency
The daily food ration (R.A) is calculated according to this formula [9] ,

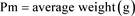 ;
; 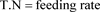
The rate and frequency of feeding at the start of the experiment were the same for all twelve batches, but the rate changed according to the average weight of the individuals; it was 25% of the biomass from day 1 to day 10, then 20% from day 11 to day 20 and finally 15% from day 21 to day 28.
The feed ration was distributed twelve (12) times a day over a period of 28 days after each parameter was taken.
3. Monitoring Physical, Chemical and Zootechnical Parameters
3.1. Physical and Chemical Parameters
The physical and chemical parameters of the water, such as temperature, pH and dissolved oxygen, are measured every day using a thermometer, a pH meter and an oximeter. Table 1 shows the physico-chemical parameters of the different rearing systems.
3.2. Zootechnical Parameters
A sample of 25% of the population was taken on a weekly basis to check the biomass and readjust the feed ration for the week. After 28 days of rearing, all the fish were sampled and counted, and 20 individuals chosen at random from each tank were measured for length and individual weight. On day 75, corresponding to the end of pre-pregnancy, all the fish were captured and sexed manually. Sex was confirmed by direct observation of the genital papillae of each specimen. Various zootechnical parameters such as sex ratio, survival rate, average weight gain, specific growth rate, nutrient quotient and mortality rate were calculated. Table 2 shows the calculation of zootechnical performance parameters.
![]()
Table 2. Calculation of zootechnical performance parameters.
4. Statistical Analysis
Sexual reversal rate, daily weight gain, weight growth and survival rate were subjected to one-way analysis of variance (ANOVA 1). The ANOVA indicated significant differences (p < 0.05). These analyses were performed using STATISTICA 7.1 software.
5. Results
5.1. Sex Reversal Rate (%)
Data on sexual inversion rates are listed in Table 3. The percentages of males in the positive control batch and the batch treated with 20 g of G. kola were significantly (p < 0.0001) higher than those in the other batches. On the other hand, that of the negative control batch was significantly lower than that of the batches treated with the food supplement (p < 0.017). For the batches treated with G. kola powder, the best male rate was obtained in the batch treated with 20 g of G. kola (94.03% - 93.06%). The greater quantity of G. kola extract, the less the individuals consumed the food because of its bitter taste (high tannin content). The comparison of the two batches (positive control and 20 g of G. kola) was significantly better than those of the other batches (negative control and 10, 30 and 40 g of G. kola). The positive control batch had 97.03% and 98.09% males respectively and the batch treated with 20 g of G. kola had 94.03% and 93.26% males respectively. The negative control batch had 49.22% and 53.02% males respectively and the batch treated with 10 g of G. kola had 80.01% and 75.72% males respectively. Table 3 shows the distribution of sexual inversion rates by basin and Figure 1, Figure 2 show sexual inversion rates as a function of treatment.
5.2. Weight Growth
Weight growth data are shown in Table 4. The treatment duration was set at 28 days. The observed weight growth performances were significantly better (p < 0.009; Friedman’s k paired samples comparison test) (Table 4) in the batches fed the positive control diet and 30 g of G. kola extract, followed by those fed diets containing 10 and 20 g of G. kola extract. On the other hand, the negative controls were closer to those containing 40 g of G. kola extract. On the 28th day of treatment, a sample of 50 individuals was taken from each tank and the mean final weights of the populations treated with 10, 20, 30 and 40 g of G. kola extract reached 0.532 - 0.588 g, 0.672 - 0.644 g, 0.868 - 0.896 g and 0.476 - 0.504 g respectively. Those of the negative and positive control populations reached
![]()
Table 3. Distribution of sexual inversion rates by basin.
![]()
Figure 1. Sex reversal rate as a function of treatment.
![]()
Figure 2. Sex reversal rate as a function of treatment.
![]()
Table 4. Weight growth performance as a function of treatment duration.
0.308 - 0.308 g and 0.896 - 0.980 g respectively. The growth performance of the inverted fry was very good. These results confirm the study according to which the incorporation of plant extracts and hormone in the diet of tilapia O. niloticus significantly improves the growth performance of fry compared to the simple commercial feed commonly used in fish farming [1] . Figure 3 shows the dynamics of tilapia weight growth according to the feed ration.
5.3. Weight Gain of Tilapia on Day 28 According to Feed Ration
On day 28, the Tilapia had the same weight gain when fed the positive control as when fed a ration supplemented with 30 g of Garcinia kola. In fact, any increase of 10 g in the amount of Garcinia kola produced a significant weight gain up to 30 g (p ≤ 0.023). Beyond this quantity, the increase in the proportion of bitter cola had a depressive effect on the weight growth of the Tilapia. Figure 4 shows the weight gain of Tilapia on day 28 according to the feed ration.
![]()
Figure 3. Dynamics of tilapia weight growth according to feed ration.
![]()
Figure 4. Tilapia weight gain on day 28 according to feed ration.
5.4. Survival Rates
Survival rates were higher when Tilapia were fed a diet enriched with Garcinia cola powder compared to survival rates obtained with fish fed Positive Control and Negative Control (p ≤ 0.039). Although the survival rate was not significantly different in Tilapia supplemented with Garcinia, the excess would appear to lead to Tilapia mortality. Thus, on day 28, the mortality rate recorded for the survival rates was close to 95% in the batch of fish treated with fish given 20 g of G. kola. On the other hand, high mortality was recorded in batches treated with the hormone 17alpha methytestosterone (MT) compared with the negative control (p ≤ 0.0002). Mortality was greater during the 28 days of hormone inversion than during fry rearing. Figure 5 shows the survival rate of fish populations in different tanks.
6. Discussion
6.1. Sexual Inversion
Populations of fish treated with the hormone 17-α-methyl testosterone and 20 g of G. kola extract recorded the highest proportions of males. According to [10] , the hormone 17-α-methyl testosterone has anabolic effects. Thus, androgen-treated Tilapia fry generally grow faster than untreated individuals, probably due to increased appetite, improved food utilization and protein synthesis. However, there are certain limitations to the techniques involved, such as: contamination of broodstock, the vigilance required in the selection and maintenance of broodstock, the requirements for a high level of control and consumer acceptance of hormonal sex-reversal fish may be limited even in countries where hormonal applications to food fish are an accepted practice. On the other hand, G. kola extracts incorporated in different fish diets, favors the increase of male proportions [1] . The results of the present study confirm the studies of several
![]()
Figure 5. Survival rate of fish populations in different tanks.
authors with a reversal rate of over 95% males. The presence of steroids in G. kola seeds could be the basis of their androgenic activity. According to [11] , various pathways are associated with functional mechanisms of phytochemicals causing both masculinization and feminization at different concentrations.
6.2. Weight Growth
The present study shows that the presence of plant extracts (Garcinia kola) in the various fish diets improves their growth. The plant extracts did not have a negative influence on growth performance except for the batch of populations treated with 40 g of G. kola extracts. In fact, the various studies have shown that the use of medicinal plants as feed supplements gives interesting zootechnical results, more specifically Garcinia kola (Gultiferae). However, the results also show poor growth in populations treated with a high concentration of Garcinia kola (40 g). G. kola is a plant rich in tannins. The tannins derived from this plant are bitter and form a high polyphenolic complex with proteins, making it unavailable in the diet. Tannin can reduce protein quality by lowering digestibility and palatability. It also inhibits the activities of digestive enzymes. The presence of a high proportion of tannins in diets enriched with higher concentrations of root or seed powder could lead to poor food consumption by fish, resulting in stunted growth. G. kola is a medicinal plant commonly used in traditional medicine, rich in flavonoids, saponins, steroids, tannins and terpenoids [12] . These bioactive principles are thought to influence various parameters, including appetite stimulation and fish growth in aquaculture. In addition, the growth of inverted fry is very impressive. [13] Compared the rearing performance of different strains of Oreochromis niloticus and found that across all strains, TM treatment of the fish resulted in a final size 10.7% larger than untreated fish. This supports the idea that treated fish populations grow much faster than those fed simple feed and is consistent with the results of our present study.
6.3. Survival Rates
In the course of this study, we recorded good survival rates for batches fed with G. kola plant extracts and also for batches treated with simple feed. On the other hand, the populations of fish treated with methytestosterone hormone were low. [12] Specifies that the mortality rate is higher in the early stages of development, during hormone treatments, and tends to stabilize in the later stages of ontogeny. In other words, concentrations of plant extracts did not have a negative influence on larval survival rates. Similar conclusions were reached by [1] . Similarly, the survival rates observed would indicate that these fry value the feed well without affecting their survival.
7. Conclusion
The use of feed supplements in the diet of fish populations improves growth performance. One of the best results recorded for growth performance and survival rate is 20 g/kg. For a concentration higher than 30 g/kg, growth envoys that of populations fed with simple food and the fry tend not to eat because of the high concentration of tannin in the food. In fact, in tanks treated with 40 g of G. kola extract, almost half of the feed was not consumed by the fish. Further studies would therefore be required to extract the tannin from the Garcinia cola powder in order to achieve the ideal requirement of 100% males and enable the fry to eat properly.