Acute and Sub-Chronic Toxicity Evaluation of the Crude Methanolic Bark Extract of Bridelia micrantha (Hochst.) Baill. (Phyllanthaceae) and Its Fraction ()
1. Introduction
The use of medicinal plants is on the rise worldwide, because of the enormous expansion of traditional medicine and a growing curiosity in herbal therapies. Plants are used in treatment to preserve and improve physical, emotional, and moral fitness or to cure particular diseases and disorders [1] . According to the World Health Organization (WHO), up to 80% of the world’s population uses medicinal plants for their primary health care. This is sometimes the only affordable and accessible source of care, especially for the poorest patients [2] . There are many types of plants in Africa in general and Cameroon in particular, used in traditional medicine to treat diseases of various origins [3] . However, despite the importance of the medicinal plant sector for the national and regional economy, their exploitation is carried out under artisanal conditions [4] . Most herbalists do not indicate the protocol usage that goes with their products; they also do not know the side effects and the toxicity of the plants used. Therefore, in-depth scientific studies on the benefits and risks of these plants could allow their efficient use to prevent producing adverse effects on the population’s health [5] .
Bridelia micrantha, commonly known as the coastal golden leaf, is a member of the family Phyllanthaceae [6] . It is a plant widely used across Africa and in Cameroon. Ethnobotanical knowledge reveals that a decoction of the bark of the trunk is generally used in Cameroon against several ailments such as amoebic dysentery, cough, eye pain, diarrhoea, gastric ulcers, infertility [7] . Previous studies on this plant showed anti-cancer [8] , antidiabetic [9] , antidiarrheal, antiplasmodial, antioxidant properties [10] . In precedent studies, we confirmed the antibacterial activity of the stem bark methanol extract of this plant [11] . They revealed a significant increase of this activity achieved with an active fraction F6 following a partition of this methanol extract and column chromatography on silica gel of the dichloromethane (DCM) portion [11] . HPLC chemical analysis of the fraction F6 highlighted the presence of almost 180 identified compounds from various secondary metabolites, including alkaloids, flavonoids, phenols, quinones, steroids and terpenoids [12] . Thus, further studies are necessary to continue exploring the antimicrobial effect of this stem bark methanol extract and its fraction. Hence, assessing their toxicity profiles is crucial; toxicity studies concerning B. micrantha extracts are still scarce, and most results are preliminary [13] . This study investigated the acute and subacute toxicity of the stem bark methanol extract of B. micrantha and the F6 fraction.
2. Materials and Methods
2.1. Plant Material
Fresh barks of B. micrantha used in this experiment were collected in January 2017 in the Centre Region of Cameroon at Mount Kalla. The plant was identified at the Cameroon National Herbarium, where a voucher specimen N˚ 5714 HNC (YA) was deposited.
2.2. Experimental Animals
Female and non-pregnant mice (Mus musculus nulliparous strain, 8 - 12 weeks old and 12 - 30 g) were used for the acute toxicity test, according to the Organization for Economic Cooperation and Development [14] . Nulliparous and non-pregnant adult rats (Wistar albino strain, 8 - 10 weeks and 100 - 180 g) were used for sub-chronic toxicity tests. Animals were obtained from the Animal Biology Laboratory, University of Yaoundé I. They were placed in plastic cages under normal laboratory conditions (12 hours light/dark cycle, 25˚C ± 2˚C) for an acclimatization period of 7 days before the experiments. They received daily a standard food ration and water. Experiments were conducted according to internationally accepted guidelines for evaluating the safety and efficacy of herbal medicines [15] .
2.3. Plant Extraction
Bridelia micrantha barks were collected and dried for 21 days under shade and ground into powder. The powdered plant material (2.5 kg) was soaked in 10 L of methanol for 3 days. The mixture was filtered using a Whatman No.1 filter paper, and the residue was re-extracted four times as previously described. The total methanol extract was concentrated using a rotatory evaporator (Heidolph). The extract was dried in an oven (VENTI-Line) at 45˚C for 24 h.
2.4. Fractionation of the Crude Extract
The extract (600 g) was dissolved in a mixture of methanol (2 L), distilled water (1 L) and dichloromethane (DCM) (2 L). The DCM phase and the methanol/water phase were separated. Each phase was concentrated using a rotatory evaporator. The DCM phase (150 g) was chromatographed through a silica gel (250 - 300 Mesh) as described previously [11] . Nine fractions labelled F1 to F9 were obtained. The fraction F6 (24 g) was used in this study.
2.5. Acute Oral Toxicity
The acute toxicity study was conducted according to OECD guidelines No. 425 [14] . Eighteen mice were divided into three groups of six animals each. They were fasted for 4 h and then weighed before the experiment. Control group received only distilled water (10 ml/kg), while experimental groups received the B. micrantha methanol extract solution and fraction F6 solution, prepared in distilled water at a single dose of 2000 mg/kg body weight (bw). Animals received substances by gavage using a probe. All animals were observed for the first 3 h for toxicity signs (pain sensitivity after tail nipping, noise sensitivity after metal shock, tail condition, stool appearance, mobility and tremors). After this time, animals were supplied with food and water, and then observed for 14 days. During this period, animal weights were recorded once per week.
2.6. Sub-Chronic Oral Toxicity Study
2.6.1. Experimental Design
This study was conducted according to OECD [15] . Thirty-five rats were divided into seven groups of 5 animals. Group 1 (control) received distilled water. Groups 2, 3, and 4 received the extract at 200, 400 and 800 mg/kg bw respectively; also groups 5, 6 and 7 received fraction F6 at 200, 400 and 800 mg/kg doses. The administration was once daily over 28 days by gavage. During the experiment, body weight was noted once every two days, and toxicity signs of the individual animals or in the community were observed every week (mobility, tremor, social interaction and sensitivity to noise).
On day 28, all animals were subjected to a 24-hour food and water fast. Animals were then weighed, anesthetized intraperitoneally with ketamine (50 mg/kg) and sacrificed. Blood was collected in EDTA and dry tubes by cardiac puncture. The blood contained in EDTA tubes was transported to the hospital for the complete blood count, while the blood contained in dry tubes was left to rest for 6 hours and then centrifuged at 3000 rpm for 15 min. The serum was collected and stored at −80˚C to determine biochemical toxicity parameters.
Animals were dissected and vital organs such as liver, heart, kidneys, lungs, spleen, and uterus were removed. These organs were degreased, rinsed in a 0.9% NaCl solution and drained. Each organ was weighed and the organ relative weight compared to fasted animal weight was calculated. Organs were then preserved in a 0.9% Formalin-NaCl solution to produce histological sections.
2.6.2. Blood Analysis
Hematological parameters such as white blood cell (WBC) count, red blood cell (RBC) count, platelet (PLT) count, hematocrit (HCT), hemoglobin (HGB), mean corpuscular volume (MCV), mean cell hemoglobin (MCH) and mean corpuscular hemoglobin concentration (MCHC) were determined using an automatic analyzer (System H1, Bayer Diagnostics). The serum was analyzed for total protein, total cholesterol, HDL cholesterol, creatinine, urea, triglycerides, glucose, and alanine transaminase (ALT)/aspartate aminotransferase (AST) activities using specific commercial diagnostic kits (Fortress Diagnostics, London, UK).
2.6.3. Histopathological Analysis
Kidney and liver samples were preserved in 0.9% Formalin-NaCl solution and processed by conventional techniques. Before microscopic examination, Paraffin sections (5 μm thick) were stained with hematoxylin and eosin.
2.6.4. Ethical Considerations
Experimental protocols used in this study strictly aligned with the internationally accepted standard ethical guidelines for laboratory animal use and care as described in the European Community guidelines, EEC Directive 86/609/EEC of the 24th November 1986 [16] . This study was approved by the Institutional Animal Ethics Committee (Registration No. 778/PO/a/03/CPCSEA; 03.09.).
2.6.5. Statistical Analysis
Data were statistically analyzed using SPSS software 16.0. Quantitative data were expressed as mean ± SD. One-way ANOVA followed by Newman-Keuls post hoc test was used for multiple comparisons, and the values of p < 0.05 were considered statistically significant.
3. Results
3.1. Acute Oral Toxicity
The acute toxicity test using an oral limit dose of 2000 mg/kg of either the crude methanolic bark extract of Bridelia micrantha or the F6 fraction caused no adverse reactions or death in mice throughout the 14 days study period. Therefore, the oral LD50 of the extract and the F6 fraction is considered greater than 2000 mg/kg in mice.
3.2. Sub-Chronic Oral Toxicity
3.2.1. Weight Gain
The extract or the F6 fraction did not significantly affect the animals’ food intake. Moreover, the animal weight generally increased over time (Figure 1).
3.2.2. Relative Organ Weights
No significant changes in organ weight (liver, kidney, heart, and spleen) were observed between the treated and control rats after oral administration of the crude methanolic bark extract of Bridelia micrantha or fraction F6 over 28 days (Table 1).
3.2.3. Biochemical Parameters
The effects of administration of B. micrantha and fraction F6 after 28 days on plasma biochemical parameters in experimental rats were presented in Table 2. No significant difference (p > 0.05) was found in albumin, total protein, uric acid, and HDL-cholesterol levels. No significant difference (p > 0.05) was noted in
![]()
Figure 1. (a) Evolution of the animals’ weight during 28 days of administration of the crude methanolic bark extract of Bridelia micrantha; (b) Evolution of the animals’ weight during 28 days of administration of the fraction F6.
![]()
Table 1. Relative organ weights after 28 days of oral administration in female rats according to doses of the crude methanolic bark extract of Bridelia micrantha or fraction F6
Data are expressed as mean ± SD, n = 5. No statistically significant difference was observed between the test and control groups. Em: crude methanolic bark extract of Bridelia micrantha; F6: fraction F6.
![]()
![]()
Table 2. Biochemical parameters in experimental animals after 28 days of oral administration in female rats according to doses of the crude methanolic bark extract of Bridelia micrantha or F6 fraction.
Data are expressed as mean ± SD, n = 5. *Indicate a significant difference at p < 0.05 compared to the appropriate control group (Newman-Keuls). Em: crude methanolic bark extract of Bridelia micrantha; F6: fraction F6.
![]()
Table 3. Hematological parameters in experimental animals after 28 days of oral administration according to doses of the crude methanolic bark extract of Bridelia micrantha or F6 fraction.
WBC: White Blood Cell Count, RBC: Red Blood Cell Count, HGB: Hemoglobin, HCT: Hematocrit, MCV: Mean Corpuscular Volume, MCH: Mean Cell Hemoglobin, MCHC: Mean Corpuscular Hemoglobin Concentration, PLT: Platelet Count, MON: Monocytes, LYM: Lymphocytes, NEUT: Neutrophils. Data are expressed as mean ± SD, n = 5. *Indicate a significant difference at p < 0.05 compared to the appropriate control group (Newman-Keuls). Em: crude methanolic bark extract of Bridelia micrantha; F6: fraction F6.
animals treated with the extract compared to the controls regarding the creatinine level. The fraction F6 at doses of 200, 400 and 800 mg/kg caused a significant (p < 0.05) decrease in creatinine level. Administration of the extract or fraction F6 in repeated doses for 28 days resulted in a significant reduction (p < 0.05) in serum ALT, AST, glucose concentrations. Similarly, compared to controls, there was a significant decrease (p < 0.05) in total cholesterol, LDL-cholesterol and triglyceride levels in animals given the extract or fraction F6.
3.2.4. Hematological Parameters
There was no significant variation (p > 0.05) independently of the dose, compared to the control group, in the animals having received the extract or fraction F6, concerning the number of erythrocytes (RBC), leukocyte count (WBC), hemoglobin count (HGB), mean corpuscular hemoglobin count (MCHC), neutrophile count (NEU), lymphocyte count (LYM), monocytes count (MON), mean cell hemoglobin (MCH), and mean corpuscular volume (MCV). Similarly, no significant difference was observed concerning the hematocrit level (HCT) in all treatments, except for groups receiving the extract at 800 mg/kg and the fraction F6 at 200 mg/kg, for which there was a significant increase (p < 0.05). There was also a significant increase (p < 0.05) in blood platelet number (PLT) in the group having received the extract at 800 mg/kg compared to the control group.
3.2.5. Liver and KIDNEY HISTOPATHOLOGY
Histological studies showed no abnormalities in the liver and kidney tissue from the rat treated with the crude methanolic bark extract of Bridelia micrantha or fraction F6 for 28 days compared to controls (Figure 2 and Figure 3).
4. Discussion
The extract or the F6 fraction administered orally at a dose of 2000 mg/kg did not cause death among the selected mice throughout the study. The extract and fraction F6 have a toxicity index of 5, according to the toxicity scale of chemical
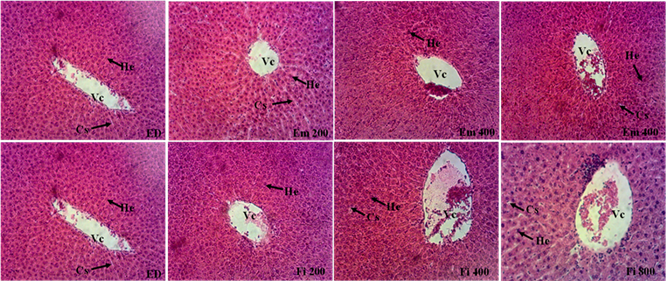
ED: control rat that received distilled water; Em 200, 400, 800, Fi 200, 400 and 800: rats that received the extract and the F6 fraction at the respective doses of 200, 400 and 800 mg/kg. Vc = centrilobular vein; He = Hepatocyte; Cs = Sinusoidal capillary.
Figure 2. Histological sections of the rat liver after 28 days of treatment at different doses of the extract and fraction F6 (Hematoxylin-eosin ×100).

ED: control rat given distilled water; Em 200, 400, 800, Fi 200, 400 and 800: rats that received the extract and the F6 fraction at the respective doses of 200, 400, and 800 mg/kg. G = Glomerulus; Eu = Urinary space; Tcd = distal convoluted tubule; Tcp = Proximal convoluted tubule.
Figure 3. Histological sections of the rat kidney after 28 days of treatment at different doses of the extract and fraction F6 (hematoxylin-eosin ×100).
substances depending on the LD50 and administration route [14] . In addition, no toxicity sign was observed during the 4 h after the extract or fraction F6 administration, particularly the decrease in sensitivity to the stimulus (pain and noise), mobility and feces softening. Onoja et al. [17] revealed that hydroalcoholic bark extract did not cause toxic effects in mice at the same dose. Some investigators, using shrimp as an animal model suggested that the plant would be toxic because of the low lethal concentrations that these authors obtained [18] [19] . However, since rodents (mice, rats) are model organisms with multiple advantages linked to their physiology, almost similar to humans [20] [21] , the results are more reliable.
The weight evolution in animals that received the extract or fraction F6 remained insignificant compared to the control during the 28 days of administration. Thus, neither the extract, much less the fraction F6, adversely affected the animals’ food intake. No significant differences in relative organ weights were found between the control and test groups. Furthermore, neither the extract nor the fraction F6 adversely affected organ weights. Aminotransferases, ALT and AST describe the cellular integrity of the liver. These are enzymes mainly synthesized in the cytoplasm of hepatic cells and discharged into the circulation when these cells are damaged. Thus, high levels of AST and ALT are frequently attributed to different drugs’ metabolic and toxic effects [22] [23] . Our results showed a significant decrease in ALT and AST levels at all doses, indicating that the extract and fraction F6 could not cause liver damage but have a hepatoprotective action. In addition, Nwaehujor and Udeh [24] showed that ethyl acetate extract from the leaves of B. micrantha had a more pronounced hepatoprotective effect than silymarin, a hepatoprotective reference molecule. Proteins are the building blocks of the human body. A high serum protein level could indicate a damaged tissue or organ. No significant difference in serum total proteins of treated animals compared to controls was observed, suggesting that the extract and fraction F6 did not cause liver damage [25] . Albumin is the most abundant protein (60%) in blood. In the body, it is produced by hepatocytes. A drop in albumin level may indicate liver failure [26] [27] . There was no significant difference in albumin levels in animals receiving the extract and fraction F6 at the different doses compared to the control group, suggesting no liver affection. Analysis of all these liver markers revealed that prolonged use (28 days) of the extract or fraction F6 had no adverse effect on liver function. This was confirmed by the histopathological examination of the liver, which showed no alteration in its structure.
Creatinine and uric acid are markers of impaired kidney function. Creatinine results from the breakdown of creatine, a constituent of muscle. Creatine can be transformed into ATP, an energy source for cells. Creatinine is excreted by the kidneys. In case of renal insufficiency, it is retained in the blood, along with uric acid, a waste product of the metabolism of purine bases. Thus, a high level of creatinine, uric acid could indicate renal failure [28] [29] . A significant decrease in creatinine levels in animals treated with the fraction F6 was observed and the values of uric acid levels did not vary significantly indicating that neither the extract nor the F6 fraction adversely affected kidney function. The histological sections of the kidneys, which present a normal architecture of the renal parenchyma, support this assertion.
The extract and fraction F6 caused a significant decrease in serum glucose levels in treated animals. This result suggests a hypoglycemic action of this extract and fraction F6. Adika et al. [30] demonstrated that the methanol extract from the leaves of this plant had an antidiabetic activity. These observations approve this plant’s use in treating diabetes in traditional medicine in several African countries [31] .
Lipids are essential for the proper body functioning. But if they are in excess, they increase the risk of cardiovascular diseases. The lipid profile helps to monitor cholesterol levels (LDL-cholesterol and HDL-cholesterol) and triglycerides [32] [33] A high LDL-cholesterol (bad cholesterol), triglycerides and low HDL-cholesterol constitute lipid abnormalities (dyslipidemias). A significant decrease in total cholesterol, triglycerides and LDL cholesterol was observed, and there was no significant difference in HDL cholesterol in treated animals. These results suggest a hypolipidemic effect of the extract and fraction F6 and could help combat cardiovascular diseases.
The analysis of blood parameters is very useful for determining abnormalities caused by plant extracts [34] [35] . It can also provide information on the mechanism of toxicity or defense of a therapeutic agent [36] . It was shown that the drop in some parameters, such as RBC, VGM, CCMH and HGB, could induce anemia [37] . The present study did not reveal any significant difference in these parameters between treated and control animals. This result indicates that neither the extract nor fraction F6 can cause anemia and has no adverse effect on the hematopoietic system.
5. Conclusion
This study aimed to assess the toxicity of the stem bark methanol extract of B. micrantha and fraction F6. Findings revealed that they did not adversely affect overall mortality and behavior in mice at tested doses. Therefore, the oral LD50 is greater than 2000 mg/kg and generally considered safe. Acute and sub-chronic toxicity investigations showed that they do not adversely affect body weight, liver and kidney, hematological and biochemical parameters. No toxicity sign was observed in the treated mice. Thus, these results showed that the stem bark methanol extract of B. micrantha and fraction F6 can be safely used in humans, and further therapeutic studies can be performed.
Acknowledgements
Authors are grateful to TCHOUPE Calixte for the reading of this manuscript; and to Pr. NJAYOU for his technical assistance.
We are forever indebted to Pr. MOUOKEU Raymond Simplice (Institute of Fisheries and Aquatic Sciences, University of Douala) for his implication in this work.