Renal Profile of Patients with Cardiorenal Syndrome: Nephrology and Cardiology Department Experience of the IBN SINA University Hospital of Rabat ()
1. Introduction
Cardio-renal syndrome (CRS) is a complex pathophysiological entity affecting the heart and kidneys in which acute or chronic dysfunction of one of the organs can induce acute or chronic dysfunction of the other organ [1] . The current classification of CRS into 5 types was proposed by Claudio Ronco’s team in 2008 [2] . It takes into account the mode of installation and the initial organ failure. A distinction is made between acute and chronic cardio-renal syndromes (CRS type 1 and 2 respectively) and acute and chronic renocardiac syndromes (CRS type 3 and 4 respectively); CRS type 5 is characterized by the association of renal insufficiency and cardiac insufficiency secondary to an acute or chronic systemic pathology. The diagnosis of these syndromes relies both on the clinical evaluation of the patients and on the measurement of specific biomarkers of cardiac dysfunction and renal dysfunction.
One in four patients hospitalized for cardiac decompensation has renal failure (RF) [3] . This RF is a risk factor for increasing the duration of hospitalization for heart failure (HF), and a risk factor for treatment failure or recurrence of HF episodes. Conversely, heart failure can lead to acute kidney failure or accentuate chronic kidney failure. CRS is always managed in a hospital setting. Clinical presentations can be severe and require invasive treatment. The objective of our study is to determine the prevalence of the different types of CRS in our patients, to define the types of renal failure, to analyze their epidemiological, clinical, therapeutic and evolutionary profile and to identify the mortality factors in our patients with a CRS.
2. Material and Method
2.1. Definitions
By the KDIGO definition, AKI is diagnosed by an absolute increase in serum creatinine (sCr), at least 0.3 mg/dL (26.5 μmol/L) within 48 hours or by a 50% increase in sCr from baseline within 7 days, or a urine volume of less than 0.5 mL/kg/h for at least 6 hours (Appendix 1) [4] .
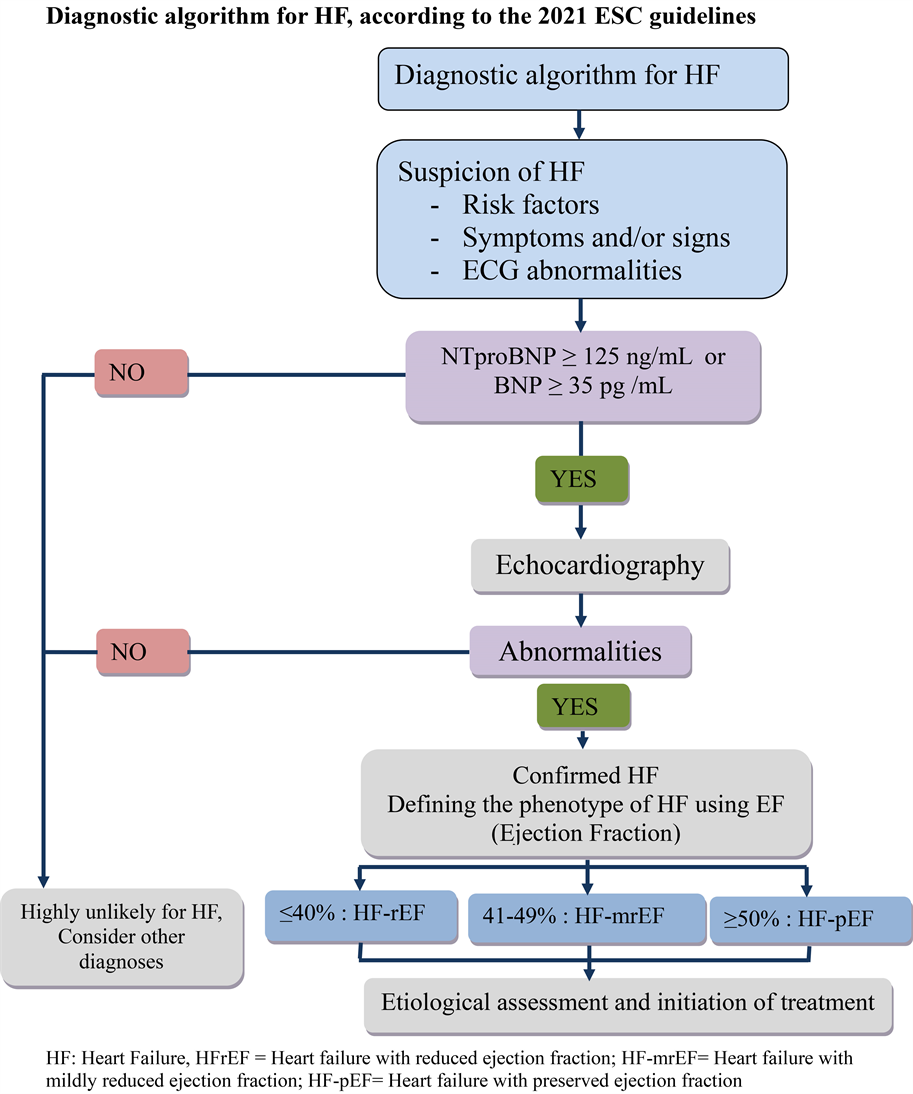
The estimated glomerular filtration rate (eGFR) is calculated using an equation from Modification of diet in renal disease (MDRD) [5] . And chronic renal failure (CRF) is defined by an eGFR of less than 60 ml/min/1.73m2 (KDIGO 2012 classification) (Appendix 2) [5] . The diagnosis of heart failure is based on clinical signs of heart failure associated with elevated biomarkers and/or systolic or diastolic dysfunction on echocardiography (European Society of Cardiology (ESC) recommendations 2021) (Appendix 3) [6] .
The definition of CRS corresponds to any acute or chronic heart failure, associated with acute or chronic renal failure. The different types of CRS are defined as follows according to the classification of Ronco and colleagues (Table 1).
The use of diuretics is defined for patients who have received one of the following diuretics without dose change for 3 days. Those who received loop diuretics are divided into 3 grades: low dose (<40 mg/day), medium dose (40 - 159 mg/day), and high dose (≥160 mg/day). Thiazide diuretics are given in 2 doses: 25 mg/day and 50 mg/day.
2.2. Study Population and Data Source
This is a retrospective, descriptive and analytical study carried out over a period of one year from June 2020 to June 2021, between the nephrology and cardiology B department and the cardiology B intensive care units of the Ibn Sina University Hospital of Rabat.
Over a year, 120 CRS patients were assessed.
Inclusion criteria: All hospitalized patients in cardiology B department for a heart condition who have a known chronic renal failure, or in whom an acute renal insufficiency is discovered during their hospitalization.
Exclusion criteria: 1) Patients with stage 5 end-stage chronic renal failure defined by an e GFR ≤ 15 ml/min (2012 KDIGO classification) not yet on dialysis, 2) chronic hemodialysis patients, 3) patients with obstructive renal failure.
The study involving human participants was approved by the ethics committee of Ibn Sina University Hospital of Rabat.
Epidemiological, anamnestic, clinical, biological, radiological and therapeutic data were obtained from the patients’ medical records. All the biological and radiological assessments were carried out at the level of the biology and radiology centers of the Ibn Sina University Hospital of Rabat.
![]()
Table 1. Classification of cardio-renal syndromes.
Judging Criteria:
The evolution of our patients is marked either by a cure defined by a clinical and biological improvement and the discharge of the patient, or by a re-hospitalization after one month, or an intra-hospital death or in the month which follows the discharge of the hospital.
A long hospital stay corresponds to a stay in the hospital for more than ten days.
2.3. Statistical Analysis
All statistical calculations were processed using Jamovi software. Quantitative variables were presented as means ± standard deviation, medians with interquartile ranges, or proportions, as appropriate. Continuous variables were compared using Student’s t test or Mann-Whitney U test. Qualitative variables were expressed as a percentage and compared using the chi-square test or Fisher’s test. A p-value less than 0.05 was considered statistically significant.
The variables were analyzed in more detail by univariate and multivariate analysis by logistic regression.
3. Results
3.1. The Different Types of SCR
Between June 2020 and June 2021, a total of 120 patients with CRS were recruited. The different types of CRS are illustrated in Table 1. CRS type 5 represents 46% of cases, and CRS types (1, 2, 4) are found respectively in (28%, 21% and 5% of cases). While CRS type 3 was not found in our patients (Table 2).
3.2. Characteristics of Patients with CRS
One hundred and twenty patients with CRS were recruited over a period of one year. All these patients were hospitalized in the cardiology department B of the Ibn Sina University Hospital of Rabat, 45% of which required a stay in the intensive care units of cardiology (CICU). The average age of our patients is 67.76 ± 12.04, with age extremes ranging from 39 years to 92 years. The sex ratio is 1.35. The history of hypertension is found in 54.2% of our patients, followed by diabetes in 41.7%. CRF was noted in 48.3% and 63% have ischemic heart disease and 16% have rhythmic heart disease (Table 3).
3.3. Renally
Clinically the mean systolic blood pressure is 130 ± 23.8 mmHg, the mean diastolic
![]()
Table 2. Prevalence of CRS in our patients.
![]()
Table 3. Characteristics of patients with CRS on admission.
CICU: cardiology intensive care unit; CRF: chronic renal failure; BP: blood pressure; CP: contrast product.
blood pressure is 81 ± 17.7 mmHg and the mean blood pressure is 97 ± 18.3 mmHg. Thirty-two percent of our patients have lower limb edema and acute pulmonary edema is found in 12.5% of cases. Acute renal failure (ARF) is noted in 51.6% of our patients with CRS, 61.3% of whom have functional ARF and 38.7% present with acute tubular necrosis. The median serum creatinine of our patients is 17.65. Chronic renal failure (CRF) is found in 48.4% of cases, of which 39% are at stage III and 61% are at stage IV. The average GFR is 29.3 ± 13.71 ml/min/1.73m2.
The etiology of CKD is dominated by hypertensive nephropathy (72.4%) followed by diabetic nephropathy (60.3%) (Table 4).
3.4. Cardiac Abnormalities in Patients with CRS
The electrocardiogram performed systematically on admission shows signs of myocardial ischemia in 47% of our patients, 30% of whom have an ST segment elevation, 64% have a Q wave of necrosis and 34% have repolarization disorders. Rhythm disorders are found in 16% of cases, and conductivity disorders in 13% of patients. Cardiac ultrasound also performed in all our patients on admission reveals hypokinesia in 40% of cases, followed by left ventricular hypertrophy in 26% of cases, and valvulopathy in 6% of cases. Signs of overload, namely the
![]()
Table 4. Types of renal damage during CRS.
ARF: Acute renal failure.
elevation of left ventricular filling pressures and the dilation of the inferior vena cava, are also objectified by transthoracic ultrasound in 26% of cases.
3.5. Therapeutic Care
On the renal level, we treated water and sodium retention with loop diuretics in 51% of cases, of which 23% received low doses, 46% received medium doses and 31% received high doses, for patients with very severe renal insufficiency we administered doses up to 1 g/d. The combination of thiazide diuretics is indicated in 21% of our patients, of whom 56% received 25 mg/day and 44% received 50 mg/day. The anti-aldosterone is administered in 12% of our patients. The monitoring of the effectiveness of diuretics is essentially done by quantifying the diuresis in certain patients who manage to collect their urine. We used conventional hemodialysis in 9% of patients with resistance to diuretics or with hyperkalemia with electrical signs on the ECG and who are resistant to medical treatment for hyperkalemia.
On the cardiac level, angiotensin converting enzyme (ACE) inhibitors are indicated in 82% of our patients and angiotensin 2 receptor antagonists in 8% of cases. Nineteen of our patients are put under the association sacubitril, valsartan. We used vasoactive drugs in 9.2% of cases. Eleven percent of patients required oxygen therapy and 7.5% required the use of noninvasive ventilation.
The evolution is marked by the discharge of 85.8% of patients. Re-hospitalization in the following month is indicated in 10.8% of cases, the causes of readmission being dominated by deviation from the diet (47%) and pneumonia (35.3%), followed by rhythm disorders (17.6%) (Table 5). The mortality rate is 14.2%.
3.6. Mortality Risk Factors
In univariate analysis, advanced age, long hospital stay, rehospitalization, acute pulmonary edema (APE), use of hemodialysis, right heart failure (RHF), presence Valve disease and hemodynamic instability are all risk factors for mortality in patients with CRS (OR = 1.15, p = 0.01; OR = 4.5, p = 0.03; p < 0.01; OR = 2.7, p = 0.023; p = 0.018, OR = 14.12; p = 0.046, OR = 14.53; p = 0.009; OR = 20.06, p = 0.002, respectively). Re-hospitalization is significantly correlated with profound anemia (p ≤ 0.01). Unlike the male sex, ARF, CRD, water and sodium retention are not significantly related to death (Table 5). In multivariate analysis,
![]()
Table 5. Logistic regression table in univariate analysis determining the mortality risk factors of patients with CRS.
ARF: Acute renal failure; CRF: chronic renal failure; HJR: Hepatojugular reflux.
advanced age, male sex, long hospital stay, APE, ARF, and the use of hemodialysis are all factors significantly associated with mortality (OR = 2.12, p = 0.021; OR = 1.26, p = 0.047; OR = 5.1, p = 0.024; OR = 1.92, p = 0.027; OR = 3.41, p = 0.034; OR = 4.3, p = 0.048) (Table 6).
4. Discussion
In this retrospective study, we analyzed the renal profile of patients with CRS, as well as their prognostic factors. CRS type 5 is predominant in our study and represents 46% of cases. In the literature, epidemiological data concerning CRS type 5 are rare and insufficient given the large number of etiologies that could potentially be responsible for this disorder [7] [8] . Sepsis in its most severe form is the greatest provider of CRS type 5, in several publications, [7] [8] contrary to what we found in our study where sepsis is not found only in 15% of our patients. Chronic type 5 CRS is found in 31% of cases in our study, the causes of which are dominated by diabetes and hypertension. Systemic amyloidosis with
![]()
Table 6. Logistic regression table in multivariate analysis determining the mortality risk factors of patients with CRS.
renal and cardiac involvement is an increasingly common cause of CRS 5. Heart and kidney amyloid deposits resulting in restrictive cardiomyopathy and proteinuria kidney disease. It is important to note that the presence and severity of CRS affect the prognosis of systemic amyloidosis. Systemic AA and AL amyloidosis are by far the most common types [9] [10] [11] . Renal involvement, very frequent and often inaugural, is observed in more than 90% of cases during AA amyloidosis and in 50% - 60% during AL amyloidosis [12] [13] [14] . Exceptional cardiac involvement during AA amyloidosis is especially the prerogative of AL amyloidosis where its frequency varies from 60% to 80% [9] [10] [13] [15] . In our study, two patients have AL amyloidosis with cardiac involvement. Several published observational studies have shown the incidences of CRS type 1 to be 25.9% - 38.9% [16] [17] [18] . This agrees with the results of our study in which the incidence of CRS 1 is 28.4%. Another study shows that CRS type 1 is present in 25% of patients admitted for cardiac decompensation in the context of chronic heart failure [17] [18] .
CRS 2 is found in 21% of our patients, which is consistent with an Australian study in which CRS 2 represents 25% [19] . CRS 3 is not found in our study since this type of CRS is generally found in patients hospitalized in the nephrology department for acute renal failure which will lead to acute heart attack, whereas our patients included in the study are all hospitalized in the cardiology department, and therefore the initial damage is essentially cardiac with renal repercussions, or the damage to the two organs is secondary to a systemic disease.
Many factors have been described in the literature as being associated with the onset of renal failure in patients hospitalized for heart disease: male gender, hypertension, pre-existing renal dysfunction, tachyarrhythmias [20] .
Some of these factors were also found in our study, mainly hypertension and a history of renal failure, while male sex was not found in our study. Conversely, advanced age, diabetes and hypertension were strongly linked to the occurrence of cardiovascular complications in patients with chronic kidney disease, according to other studies, which is consistent with the data from our study [21] [22] . On the other hand, smoking is not correlated with the occurrence of cardiovascular events in our patients, contrary to what is reported in certain studies [23] .
The reduction in cardiac output, during CRS 1, secondary to the cardiac aggression generates renal hypo-perfusion with an increase in venous pressure leading to renal congestion, explaining the suffering and renal damage, leading to a drop in flow. glomerular filtration and installation of an ARF.
Heart disease is particularly common in patients with chronic kidney disease (CKD), with a major impact on the survival of this population. A patient with chronic renal failure is more likely to die from cardiovascular disease than to reach the stage of dialysis [24] . Cardiovascular events are twice as frequent when the glomerular filtration rate (GFR) is between 45 and 30 ml/min, and 2.8 times more frequent when the GFR is between 30 and 15 ml/min compared to patients having no CKD [24] . If CRMs in themselves promote the development of heart disease, mainly left ventricular hypertrophy, heart failure and coronary artery disease, diabetes and arterial hypertension, as causal or associated pathologies of renal failure, are also directly involved [25] . In our study, 54% of our patients with CRS have hypertension and 42% have diabetes. In CKD, several predisposing factors are combined for the development of LVH, the prevalence of which in patients with CKD increases with the decline in renal function ranging from 26.7% with a CrCl greater than 50 ml/min to 45, 2% when CrCl is less than 25 ml/min [26] . Hypertension is a risk factor for LVH and mortality in CKD. Rigorous treatment of hypertension can lead to the reduction of left ventricular mass [27] . Angiotensin-converting enzyme (ACE) inhibitors are thought to be more effective than other antihypertensives in achieving this effect [28] . In our study, 26% of patients had LVH. CRF anemia mainly results from decreased erythropoietin (EPO) production. This anemia causes vasodilation and an increase in cardiac output, thus constituting a hyperdynamic circulatory state that contributes to LVH and heart failure on the one hand and to arteriosclerosis on the other. Apart from CKD, anemia is also a risk factor for mortality in heart failure [29] [30] .
This relative risk is doubled if the anemia is accompanied by a clearance equal to or less than 30 ml/min. Correction of anemia with erythropoietin results in improved ventricular function and renal function [30] . It is considered one of the causes of LVH and predisposes to ischemic heart disease. Its correction breaks the cardio-renal vicious circle allowing at the same time a regression of cardiac and renal dysfunctions. Several studies have shown, in heart failure patients with moderate CRF, an improvement in their prognosis and a reduction in hospitalizations after stabilization of hemoglobin around 120 g/l [31] .
Phosphate retention begins early in CRF when the decrease in GF reduces the filtered phosphate load [32] . The resulting elevation of calcium phosphate product contributes to valvular and vascular and extravascular calcifications [33] . Early correction of phosphocalcic disorders during CKD can prevent or even reduce the risk of these complications.
Diuretics are the first-line treatment for CRS with fluid and sodium retention. In an algorithm summarizing bulk management in heart failure patients with refractory CRS advocates using increasing doses of loop diuretics in the first line of treatment for bulk removal, with combined diuretic therapy used for patients who do not respond adequately to loop diuretics [34] . A target urine output of 3 - 5 L/day has been used in studies of progressive diuretic therapy [35] [36] . Hemodynamic assessment and vasoactive therapy may be helpful in some patients with poor response to diuretics, with extracorporeal volume suppression for patients whose medical treatment fails (Appendix 4).
A study comparing the use of continuous versus bolus diuretics in patients with cardiac decompensation did not find any difference in terms of efficacy [37] . What we also found in our study. The AVOID-HF (Aquapheresis versus Intravenous Diuretics and Hospitalization for Heart Failure) trial tested the hypothesis that patients hospitalized with heart failure treated with ultrafiltration would have a longer time to first heart failure event in 90 days after discharge from hospital than those receiving intravenous loop diuretics [38] . Veno-venous ultrafiltration is a therapeutic alternative in this context. Potential benefits of ultrafiltration include better control of fluid removal rate and volume, greater net sodium loss, and less neurohormonal activation. Current treatment guidelines indicate that ultrafiltration is a reasonable approach in patients with congestion who are unresponsive to medical treatment. However, little is known about the safety and efficacy of ultrafiltration compared with pharmacological therapy in patients with acute decompensated heart failure complicated by acute cardiorenal syndrome and persistent congestion. Therefore, the Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARRESS-HF) was conducted to compare the effect of ultrafiltration with that of stepped pharmacological treatment on renal function and weight loss in patients with heart failure who have worsening kidney function and persistent congestion. The use of a tiered pharmacological treatment algorithm was found in the CARRESS-HF study to be superior to an ultrafiltration strategy for preservation of renal function, with similar 96-hour weight loss with two approaches. Ultrafiltration was associated with higher rates of adverse events [39] .
In a meta-analysis comparing the use of diuretics and the use of ultrafiltration in patients with volume overload in decompensated heart failure, there was no difference in mortality in the two groups of patients [40] . Whereas in our study the use of hemodialysis is found as a risk factor for mortality in multivariate analysis.
Chronic renal failure is found in 45% of our patients with chronic heart failure and is associated with an increased risk of hospitalization and mortality. This risk is all the more important as the severity of the renal insufficiency is important, we can speak here of a gradient of risk of hospitalization and mortality according to the alteration of the glomerular filtration rate (GFR) [41] .
Several poor prognostic factors associated with a high risk of mortality in patients with CRS have been described in the literature: acute pulmonary oedema, oliguria, hyponatremia, increased serum creatinine, resistance to diuretics and the need for hemodialysis. In our study advanced age, long hospital stay, CRS type 1, right heart failure, presence of valvulopathy, use of oxygen therapy and vasoactive drugs were all associated with high risk, mortality in patients with CRS. Mortality is not significantly higher in our anemic patients, contrary to what is described in several studies. The increase in left ventricular ejection fraction was a protective factor in some studies, while the absence of hypertension is the only protective factor against mortality found in our study.
5. Conclusion
CRS type 5 was most common, with hypertension and diabetes being primary causes of Chronic Kidney Disease. Mortality factors were linked to acute pulmonary edema, hemodialysis, right heart failure, valvulopathy, and re-hospitalization.
Appendix
Appendix 1. AKI Definition and Staging According to KDIGO Criteria
sCR = serum creatinine, eGFR = estimated glomerular filtration rate.
Appendix 2
Estimated glomerular filtration rate (eGFR) is calculated using a Modification of diet in renal disease (MDRD) equation.
And chronic renal failure (CKD) is defined by an eGFR less than 60 ml/min/ 1.73m2 (2012 KDIGO classification).
CRD: chronic renal disease, GFR: glomerular filtration rate. *Kidney damage manifested by histological or/and biological or/and morphological abnormalities.
Appendix 3
The diagnosis of heart failure is based on the recommendations of the European Society of Cardiology (ESC) 2021.
Appendix 4
Table: Volume management in patients with CRS [28] .