The Association between Vitamin D Deficiency and Developing COVID-19 Related Serious Illnesses—A Systematic Review and Meta-Analysis ()
1. Background
Coronavirus is rapidly transmitted and highly infectious, and it is also associated with high morbidity and mortality [1] . However, there are no actual drugs approved for the treatment of coronavirus. Anti-inflammatory drugs, antiviral drugs, neuraminidase inhibitors, Chloroquine, remdesivir are all used as a choice of drug for treatment of COVID-19 patients [2] [3] . Also Oxygen, anticoagulant, and prolonged mechanical ventilation are used for the management of COVID-19 patients [4] . However, all of these are for symptomatic treatment and there is a chance that it can develop drug resistance. Therefore, it is needed to protect people by enhancing the immune system or protective mechanism of the human body. Furthermore, Vitamin D has a mechanism to enhancing the innate and adaptive immune system which can reduce the risk of infection and mortality [5] .
The coronavirus infection occurs via angiotensin-converting enzyme 2 (ACE2) [6] . Additionally, in the respiratory tract, most of the ACE2 formation occurs [7] ; thus the respiratory tract is mainly infected by coronavirus than other systems of the body. On the other side, Vitamin D produces an immune response against various viruses, which cultured epithelial cells of respiratory systems [8] [9] .
The laboratory investigations have shown that in the serious COVID-19 patients significantly high pro-inflammatory cytokines development occurs which is called “cytokine storm” [10] . Similarly, the evidence suggests that Vitamin D can decrease the over cytokine production by enhancing the innate immune system of the body [11] . Therefore, Vitamin D can make an intervention in COVID-19 related serious illness.
2. Method
2.1. Search Strategy
The systematic search for eligible studies was carried out on published literature and data were obtain from different studies based on the association between Vitamin D deficiency and developing COVID-19 related serious illnesses. A computerised comprehensive literature search was performed via using PubMed, CINAHL and Google Scholar. Search terms and key words were defined by the researcher related to vitamin D and COVID-19 which were included in the title or the abstract: (“Vitamin D” OR calciferol OR calcitriol OR “25(OH)D” OR “25-hydroxyvitamin D” OR “hydroxycholecalciferols” OR “hypovitaminosis D” OR “micro-nutrient”) AND (COVID19 OR coronavirus disease OR “2019-nCoV” OR “coronavirus disease 2019”). The articles were independently reviewed by the researcher which published between January 2020 and January 2023. All the articles were included which was published in English and others language studies with English summary. Some articles were also included through bibliography of other related articles and by experts or senior authors which meet the eligible criteria.
2.2. Inclusion Criteria
It considered literature to be eligible which was published observational or interventional study with any age group of population and if it compared patients with COVID19 vs non COVID-19 or serious illness in confirmed COVID19 patients with or without vitamin D supplement at any dose or form. Eligibility characteristic of a literature were defined before performing the literature search on basis of PECO/PICO strategy (patient, exposure/intervention, comparison/control, outcome—the strategy which helps in the formation of the research question and search for evidence). According to the PECO/PICO strategy eligibility criteria:
· Patients, population or problem: confirmed COVID-19 patients.
· Exposure/Intervention: Vitamin D deficiency/Vitamin D supplementation.
· Comparison: control/placebo.
· The outcome of the study: COVID-19 related serious illness.
2.3. Exclusion Criteria
Exclude the published studies such as reviews, case series, case reports, abstracts, comments, posters, editorial reviews, in vitro studies, duplicate publications, non-research letters, if full-text was not accessible or the study which did not mentioned a specific outcome. Ecological studies were excluded because these studies did not measure Vitamin D levels. Furthermore, research was excluded if there was an absence of a placebo or control group.
2.4. Study Selection Process
Initially, database search results were merged and duplicate studies were manually removed. After duplicates removed, articles were reviewed by the title and abstract of the paper and if unrelated to the comprehensive study question, the results of interest were excluded. The selected literatures were then assessed based on the full text. The aim was to include all studies evaluating Vitamin D level in COVID-19 positive and negative patients and to include all studies assessing Vitamin D supplementation in COVID-19 positive patients at ICU admission and deaths regardless of dosage and form. Finally, potentially eligible literatures were independently identified and selected based on inclusion and exclusion criteria. Studies were selected on the basis of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) guidelines.
2.5. Study Identification
Through the electronic data base PubMed, CINAHL and Google Scholar searched total 1243 related studies. 413 studies were identified as duplicates and excluded. After that due to unrelated topics 677 were removed. Than among this studies full text, abstracts were searched with maintaining inclusion and exclusion criteria and research question, 16 studies were found for full text. Among these studies, 10 studies were excluded after viewed full text because 2 studies did not complete the data, 7 studies did not meet the inclusion exclusion criteria and inappropriate sample found in 1 studies. Finally, 6 studies found eligible for the research. Among the 6 studies 2 are randomised control trials and 4 are case control studies. A detailed PRISMA chart for searching is represented as Figure 1.
![]()
Figure 1. A PRISMA flowchart showing the study selection and identification process.
2.6. Data Extraction
Data extraction was done in a standardised data collection table. When the trials had more than two comparisons, only topic of interested data was collected. It was carefully checked for overlapping and the following data were recorded: title, study name, publication year, study type, country, sample size, study design and outcomes.
2.7. Evaluation of Study Quality
The quality of all observational studies was assessed by outcomes. Risk of bias and methodological quality of the eligible case control studies were evaluated by Newcastle-Ottawa scale (NOS), which evaluated the three main factors: selection of the participants, comparability of the study groups, and assessment of outcome or exposure [12] . Each included study was given an eventual point out of maximum nine points. There are four points for selection, two points for comparability and three points for outcomes [12] . High quality studies require a 7 or higher points, medium quality studies require a 4 - 6 points, and 0 - 3 points are considered poor quality. In this study four observational studies were included where three scored 7 and one scored 6 (Figure 2(a)).
![]() (a)
(a) ![]() (b)
(b)
Figure 2. (a) Quality assessment of the four included case control studies by using Newcastle-Ottawa scale (NOS). (b) Quality assessment of the two included randomized controlled trials by using Jadad score tool.
Methodological quality of the randomised control trial was assessed using the Jadad score, which represents the quality of the study based on the explanations of randomisation, blinding, and dropout/withdrawals [13] . It is known also as Oxford quality scoring system. The Jadad scoring ranges from 0 to 5 points where two or less points are considered as a poor quality study, three or more points indicate a higher quality study [13] . Jadad scale asked mainly following three questions, whether the study was described as randomised, whether the study was double blinded, whether there is present of withdrawals or dropouts’ information [13] . Each answer contains one point without any fraction. Each question had to be answered “yes” or “no”. In this study two randomised control trials were included where one scored 3 and other 5 (Figure 2(b)).
2.8. Characteristics of the Studies
Six literature sources were selected for systematic review and meta-analysis. Among them four are case control studies and remaining two randomised control trials. The main characteristics of the literatures are showing in Table 1. The included studies were conducted in China, Brazil, Iran, South Korea and 2 in Spain. All these studies were done in a clinical setting within February to October in the year of 2020. The studies were conducted in the adult population; no paediatrics or children were included. The mean age is ranging from 40 to 80 years. The proportion of women ranges from 38% to 63%. The studies were conducted over a period of 1 to 4 months. In all studies, patients with COVID-19 were diagnosed by real-time polymerase chain reaction (RT-PCR), with the exception one of the study that mentioned only about the throat swab samples. One study mentioned PCR with CURB65 severity scale and another one mentioned PCR with computed tomography scan. The studies use different laboratory procedures to evaluate participants’ Vitamin D levels such as electrochemiluminescent immunoassay (ECLIA) with a Roche Elecsys 10,100/201 system, enzyme-linked immunosorbent assay method, validated liquid chromatography-tandem mass spectrometry method, automated electrochemiluminescence system and automated competitive chemiluminescence assay. All the interventional studies, patients received supplementation of Vitamin D orally. One of the study treatment groups were supplemented 0.532 mg of calcifediol during admission then after 3 days 0.266 mg and then another on the 7th day; after the 7th day it was continued as a weekly dose. Another study interventional group received 200,000 IU single dose of vitamin D3. Furthermore, in the other study 10 patients received 25,000 IU cholecalciferol monthly basis, one patient received 5600 IU per week and remaining 8 patients consumed 0.266 mg calcifediol per month. In the studies, age and sex were assimilated in both groups, thus eliminated these confounding factors. Three studies mentioned about the randomisation.
Total participants of the studies are 1276, (participants of the individual study ranging from 76 to 402). Among them 845 are COVID-19 positive patients, 431 are COVID-19 negative patients. In addition, three studies compared COVID-19
Table 1. Characteristics of included studies for the meta-analysis.
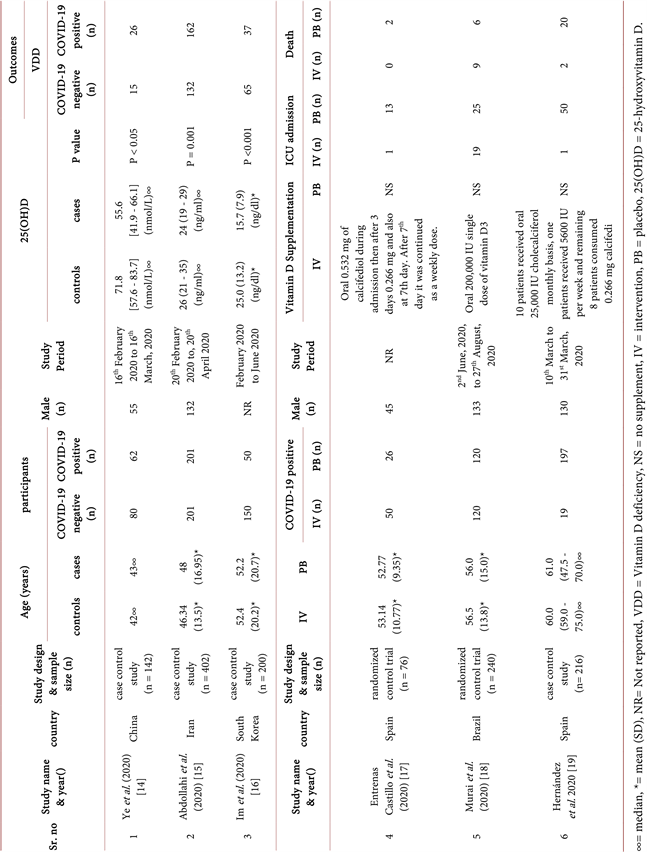
positive patients with healthy individual for determined the Vitamin D deficiency status. These three studies contain total 744 participants where 313 COVID-19 positive patients. The other three literatures are interventional studies where total participants are 532, and all are COVID-19 positive patients. Among them, 189 participants are in Vitamin D supplement arm and 343 are non-supplement arm. The first three studies result revealed that among COVID-19 patients a total of 225 have suffered from Vitamin D deficiency which is 71.88% and in healthy individuals a total of 212 are suffered from Vitamin D deficiency which is 49.19%. Furthermore, three study reports showed that a total of 21 patients with Vitamin D supplements needed to be admitted to the ICU which is 11.11%, and without Vitamin D supplements, 88 patients needed ICU admission which is 25.66%. Furthermore, three studies did not report any deaths but interventional studies mentioned the death in their studies. The total number of deaths in the Vitamin D supplement arm is 11, and the total deaths without Vitamin D supplement arm is 28 which are 5.82% and 8.16% respectively. One of the studies found in serious cases of COVID-19 a remarkably greater rate of comorbid kidney failure than in mild or moderate cases. This study also reported that in serious COVID-19 cases a remarkably higher rate of Vitamin D deficiency noted compared to mild and moderate cases. Another study found that 44.4% COVID-19 patients have selenium deficiencies. The mentioned comorbidities of participants of included studies are shown in Table 2.
2.9. Statistical Analysis
All data for meta-analysis were performed using a computer software program RevMan 5.4 (The Cochrane Collaboration, Review Manager, Oxford, Copenhagen, Denmark). The software program was used for blending the statistical data and deriving cumulative result of the intervention on concluding outcomes of interest. To analyze dichotomous data, the Mantel-Haenszel method was performed and an odds ratio was conducted for outcome evaluation with 95% confidence interval and random effects was used. I2 represents the different level of heterogeneity (high: 75%, medium: 50% and low 25%). Funnel plot was reported the publication bias. Forest plot represented: Vitamin D deficiency among healthy individuals and COVID-19 positive patients and treatment effect of Vitamin D on COVID-19 patients.
3. Result
The systematic review and meta-analysis included six studies where there are two randomised control trials and four case control studies. The meta-analysis showed that there is statistically significant (P < 0.00001) Vitamin D deficiency occurred in COVID-19 patients as compared to healthy individuals (odd ratio = 2.65 [1.88, 3.73], CI 95%, P < 0.00001) (Figure 3(a)). It also observed that without Vitamin D supplemented COVID-19 patients needed ICU admission at a higher rate compared to Vitamin D supplemented COVID-19 patients (odd ratio 0.16 [0.02, 1.45], P = 0.10, CI 95%) (Figure 4(a)). Heterogeneity found in this part of analysis but withdrawal of Entrenas Castillo et al. (2020) [17] has showed significantly decreased the heterogeneity which revealed I2 of 48% (Figure 4(c)). In this section fixed effect revealed significant statistical strength (P = 0.0003) (Figure 4(d)). In addition, meta-analysis for mortality evaluation was also done between the Vitamin D supplemented and non-supplemented COVID-19 patients. But there had been no impact on mortality found through comparing group (odd ratio= 0.98 [0.32, 2.95], P = 0.97, CI 95%) (Figure 5(a)). Publication bias was evaluated and visually represented by using Funnel plot (Figure 3(b), Figure 4(b), Figure 5(b)).
![]()
Table 2. Comorbidities related to seriousness of COVID-19 infection.
COVID-19 “D” = COVID-19 patients with vitamin D supplementation, COVID-19 “P” = COVID-19 positive, COVID-19 “N” = COVID-19 negative, ∞ = Chronic obstructive pulmonary diseases, α = Asthma, NM = Not mentioned, (NB-Im et al. (2020) study not mentioned any comorbidity).
![]() (a)
(a)![]() (b)
(b)
Figure 3. (a) Forest plot of COVID-19 positive and negative individuals showed more vitamin D deficiency occurred in COVID-19 positive patients compared to COVID-19 negative individuals. (b) Publication bias was visually represented by the Funnel plot.
![]() (a)
(a)![]() (b)
(b)
Figure 5. (a) Forest plot of COVID-19 positive patients showed no significant different in mortality among vitamin D supplemented and non-supplemented group. (b) Publication bias was visually represented by the Funnel plot.
4. Discussion
COVID-19 related serious illnesses have developed through various stages. Upper respiratory system infection with fever, body aches, weakness, diarrhoea, headache, coughing and a painful throat are all symptoms for the first stage of COVID-19 infection; however, some people may remain asymptomatic. Pneumonia and severe coughing are symptoms of the second stage, while the third stage is COVID-19 infection induced complication such as septicaemia, severe restlessness, respiratory failure, cardiovascular complication and multiple organ injury. The final stage is death or recovery [20] . In this study, all the stages of COVID-19 were investigated to check its relationship with Vitamin D deficiency. The first stage was investigated by comparing the COVID-19 positive patients to healthy individuals. The second and third stage was evaluated by comparing the COVID-19 patients with and without Vitamin D supplementation and the rate of ICU admission. The fourth and final stage is evaluated by mortality rate among Vitamin D supplement and non-supplement groups.
The study found that significant Vitamin D deficiency occurred in COVID-19 patients compared to healthy individuals (odd ratio = 2.65 [1.88, 3.73], CI 95%, P < 0.00001). A cross sectional study was done in India which also reported a significant association between COVID-19 patients with Vitamin D deficiency. It found 58.97% Vitamin D deficiency and 89.1% Vitamin D insufficiency among COVID-19 patients [21] . A study done by Mamani et al. (2017) found out that COVID-19 patients have 4.16 times more severe Vitamin D deficiency occurring than the control group [22] . A study conducted by Pinzon et al. (2020) in Indonesia which documented 90% Vitamin D (<20 ng/mL) deficiency and 10% (<30 ng/mL) insufficiency in the study group [23] . Also Kaufman et al. (2020) found out that there is higher COVID-19 positivity in lower levels of Vitamin D individuals [24] .
The conducted study also found that without Vitamin D supplemented COVID-19 patients needed ICU admission at a higher rate compared to Vitamin D supplemented COVID-19 patients (odd ratio 0.16 [0.02, 1.45], P = 0.10, CI 95%). The University of Cincinnati Health system supports these study results by conducting their own study where they found a significant association between COVID-19 related serious illnesses with Vitamin D deficiency [25] . A study revealed that reduce Vitamin D levels would impair the clinical outcome of COVID-19 patients and raise Vitamin D improved clinical outcomes of the patients [26] . Furthermore, as documented in the New York study, COVID-19 patients treated with high doses of Vitamin D where only one patient needed ICU support and the rest of the patients took only standard management [27] .
It should be noted that several studies showed no significant difference between Vitamin D deficiency and mortality. This conducted study also does not find any significant relation between mortality rate and Vitamin D deficiency (odd ratio = 0.98 [0.32, 2.95], P = 0.97, CI 95%). A study conducted with 109 COVID-19 patients where 60% were males and 40% females, aged between 44 and 78 years. This study supported Vitamin D deficiency being associated with seriousness of COVID-19 patients but it did not find any association between Vitamin D deficiency and mortality rate [28] . Also Baktash et al. (2020) which did not find any difference in the death rate between the patients who were suffering from Vitamin D deficiency and those who were within the normal limit [29] . A review conducted by Farid et al. (2021) concluded with the decision that Vitamin D can reduce the risk of acute viral infection in the respiratory tract and pneumonia via reduced viral replication, regulatory function of the immune system and anti-inflammatory effects. It reported the different beneficial effects of Vitamin D supplementation but cannot make any decision about association of Vitamin D with COVID-19 infection related mortality [30] .
Martineau et al. (2017) reported that Vitamin D is more effective in respiratory tract infection if it is supplemented weekly or daily doses. However, Bolus doses are not as effective when given daily or weekly [31] . Also another study concluded that daily or weekly doses are more protective and enhance the immune system than high bolus doses [32] . A randomised control trial found that monthly doses of Vitamin D supplementation decreased the rate of respiratory tract infection [33] . But there is no actual or definitive dose of vitamin D is recognized.
Future research could investigate the optimal dosage and duration of Vitamin D supplementation for COVID-19 patients at different stages of infection. Additionally, studies could explore the interplay between Vitamin D levels and other factors influencing disease severity, such as genetics, comorbidities, and immune response, to provide a more comprehensive understanding of the relationship between Vitamin D and COVID-19 outcomes.
This systematic review has impressive outcomes, but with some unavoidable limitations. The study was limited by the availability and character of primary research. This study included only 2 randomised controlled trials and 4 case control studies with a short time frame
5. Conclusion
COVID-19 transmits human to human very rapidly through respiratory system and its incidence and prevalence rates are very high. Furthermore, COVID-19 can produce serious illnesses which can eventually lead to death. There are no recognised drugs which are available to control these situations; however, Vitamin D has some preventive effects in respiratory system infection and has a property to enhance the immune system. In this study, the result reported that Vitamin D deficiency is common in COVID-19 patients and also Vitamin D deficiency is associated with COVID-19 related serious illnesses. So the use of Vitamin D as a preventive measure as well as treatment protocol can be beneficial to the patients. Respiratory tract infection is the main cause of seriousness and deaths of the patients. Vitamin D is cost-effective and has some good effective properties against COVID-19 infection; therefore, it is highly recommended to add Vitamin D for the treatment and preventive protocol of COVID-19. This study result has high statistical power, but there is not enough evidence documented at present time. In addition, there are not enough randomised control trials being conducted about this topic. Observational studies are not strong evidence for making any proper decisions, so it is highly recommended to do more randomised control trials for the establishment of this topic strongly.
Funding
The research did not receive any funding.