Prevalence of Some World Health Organization Priority Organisms from an Abattoir at Kwata, Anambra State, Nigeria ()
1. Introduction
An abattoir is a place where animals are killed and prepared for traders and consumers to buy for sale and consumption [1] . Over 150 million people in Nigeria receive domestic meat supplies from the abattoir, which also offers employment opportunities for the country’s teeming population. In Nigeria, abattoirs are typically found close to metropolitan areas, and significant amounts of waste generated there are dumped directly into the rivers [2] [3] [4] .
Twelve families of bacteria that pose the greatest threat to human health are included on a list of antibiotic-resistant “priority pathogens” that the World Health Organization (WHO) issued in 2017. Based on the urgency and necessity for new antibiotics, the list has been separated into three main priorities. The list was created as part of WHO efforts to address the rising global resistance to antimicrobial medications in order to direct and stimulate research and development of new antibiotics. Multidrug resistant bacteria, namely Acinetobacter, Pseudomonas, and several Enterobacteriaceae are listed among the most dangerous group of all called the “Critical group”. They are capable of causing serious infections that are frequently fatal, like pneumonia and bloodstream infections. The most effective antibiotics for treating multi-drug resistant bacteria, such as carbapenems and third generation cephalosporins, also have confirmed failed treatment outcomes. The other groups are the high and medium priority categories. The “high priority group” includes organisms such as Enterococcus faecium, Campylobacter spp., Helicobacter pylori, Staphylococcus aureus while the “medium priority group” includes Staphylococcus aureus, Haemophilus influenzae, Shigella spp. [5] .
As the demand for meat and meat products is increasing, it is important to assess the level of contamination of meat parts and meat contact surfaces in municipal abattoirs with pathogens of public health significance [6] . Information on the hygiene status of meat production areas will facilitate the development of prevention strategies for microbial contamination in abattoirs and provide baseline data for related studies [7] . The aim of the study is to identify microorganisms from Kwata meat market, listed as W.H.O priority organisms.
2. Materials and Methods
Study design and area
The study area was the Kwata meat market which is located in Awka South L.G.A, Anambra State, Nigeria. Kwata meat market has a Latitude of 6˚21'N and Longitude of 7˚05'E. Kwata meat market is a major source of meat distribution in Awka Anambra state and the study was from October 2022 to December 2022.
Sample collection and examination
A total of thirteen samples were collected from the abattoir using sterile swab and specimen bottles. The sample size was determined by the number of cows slaughtered at the time of visit to the abattoir for sample collection. Samples were taken from floor, slaughtering table, butchering knives, waste water, soil, and meat. The samples were taken to the Microbiology laboratory of Faculty of Pharmaceutical Sciences Chukwuemeka Odumegwu Ojukwu University, Igbariam Campus Anambra State, Nigeria for analysis.
Sample collection criteria
The selection criteria and selection process included all animals already slaughtered at the time of visit to the abattoir for sample collection.
Sample preparation and dilution
All the media used in the present study were prepared according to the manufacturer’s specification, and collected samples were inoculated into plates and incubated at 37˚C for 24 - 48 hours. Each sample was shaken in 1 ml of distilled water, and was diluted in 9 ml of distilled water. Ten fold serial dilutions of the homogenates were made before they were aseptically inoculated onto Petri plates using the spread and streak plating methods.
Microbiological analysis
The swabs were streaked on cetrimide agar and MacConkey agar and incubated at 35˚C - 37˚C for 24 - 48 hours. Changes in physical appearance in differential media and enzyme activities of the organisms were observed. Gram reaction and other biochemical tests namely: Indole, Citrate, Catalase and oxidase tests were also done for identification of the isolates using methods described by [8] .
Antibiotics susceptibility testing
The susceptibility tests were performed following the method M2A6 disc diffusion method as recommended by the Clinical and laboratory standards institute (CLSI, 2016) using Mueller-Hinton agar. The isolates were sub-cultured onto Mueller-Hinto agar plates and incubated at 37˚C for 18 - 24 hours. The density of suspension was determined by comparison with McFarland 0.5 Barium sulphate solution. The standardized inocula were swabbed onto Mueller-Hinton agar plate and the discs were placed on the inoculated plates. The isolates were tested against the following discs; Ofloxacin (5 μg), Amoxicillin-clavulanate (30 μg), Ceftriaxone Sulbactam (45 μg), Gentamicin (10 μg), Nalidixic acid (30 μg), imipenem/Cilastatin (10/10 ug), Ampiclox (10 ug), Levofloxacin (5 ug), Cefotaxime (25 ug), nitrofurantoin (300 ug), Cefuroxime (30 ug), Cefexime (5 ug). The plates were incubated at 37˚C for 18 - 24 hours and inhibition zone diameters were measured in millimeter
Test for biofilm formation
A qualitative method for biofilm detection as described by Christensen et al. [9] was used. A loopful of test organisms was inoculated in 3 mL of trypticase soy broth with 1% glucose in test tubes. The tubes were incubated at 37˚C for 24 h. After incubation, tubes were decanted and washed with phosphate buffer saline (pH 7.3) and dried. Tubes were then stained with crystal violet (0.1%). Excess stain was washed with deionized water. Tubes were then dried in inverted position. The scoring for tube method was done according to the results of the control strains. Biofilm formation was considered positive when a visible film lined the wall and the bottom of the tube.
3. Results
Bacteria profile
A total of 75 isolates were obtained which are all Gram negative organisms; E coli, Klebsiella spp., Proteus spp. and Pseudomonas aeruginosa. Table 1 shows the morphological characteristics of the organisms on MacConkey and centrimide agar. The frequency and distribution of the isolates are on Table 2 while Figure 1 and Figure 2 are the antibiotic susceptibility results of the isolates on conventional antibiotics. The biofilm formation test showed medium to strong biofilm forming of all the test organisms (Figure 3 and Figure 4).
4. Discussion
World Health Organization (WHO) priority organisms have caused a range of infections in man today. The research was aimed to identify WHO Priority organisms that can be found in the abattoir. Multidrug resistant bacteria, which remain a major cause of antibiotic treatment failures in hospitals, nursing homes, and among patients, are listed among the critical and high priority organisms [5] .
Bacterial contamination was identified in all the areas samples were collected from the abattoir. Adebowale et al. [10] reported that the water used for cleaning procedures and meat processing in the abattoir must meet drinking water standards. In a study by Endale and Hailay [11] , they observed that high microbial load on the knife and cutting table is an indication of inadequate cleaning. The knives are washed with water only without any other form of cleaning or sterilization. The bacteria load on the slaughter knives grow continuously as a result of multiple handling by the butchers on dirty or contaminated surfaces.
A total of 75 isolates were obtained in this study, the frequency and percentage distribution are: E. coli 9.3% (22), Klebsiella spp. 26.7% (20), Proteus spp. 16% (12), and Pseudomonas spp. 28% (21) (Table 2). This statistics agrees with a study done by Gul et al., [12] where the percentage frequency was Escherichia coli (25%), Proteus spp. (12.5), Klebsiella spp. (12.5%) and Pseudomonas spp. (18.75%). Although Escherichia coli are unavoidable meat contaminant, the numbers are usually low when good hygiene is practiced [13] Uzoigwe et al., [7] , in a similar study identified E. coli as the dominant bacteria isolate found in the abattoir. This report was also confirmed by Gurmu and Gebretinsaen [14] and Bersisa et al. [6] .
The high rate of Pseudomonas spp. contamination of meat indicates the
![]()
Table 1. Colony features and biochemical test results for MacConkey and Cetrimide Agar.
KEY: IND-Indole test; CIT-Citrate utilization test; CAT-Catalase test; OXI-Oxidase test; PO-Probable Organism; + Positive; − Negative.
![]()
Table 2. Percentage frequency of probable organisms from isolates.
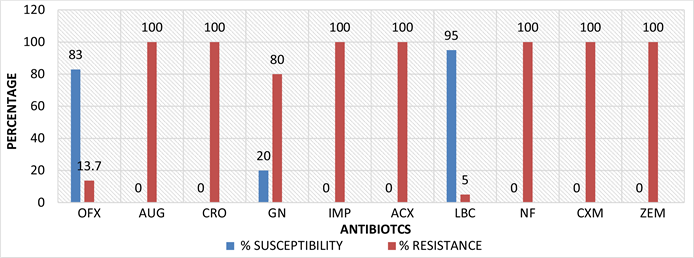
KEY: OFX: Ofloxacin, AUG: Amoxicillin-clavulanate, CRO: Ceftriaxone Sulbactam, GN: Gentamicin, IMP: Imipenem/Cilastatin, ACX: Ampiclox, LBC: Levofloxacin, NF: Nitrofurantoin, CXM: Cefuroxime, ZEM: Cefexime.
Figure 1. Percentage susceptibility test results of the isolates (Klebsiella spp., Proteus spp., E. coli).

KEY: OFX: Ofloxacin, AUG: Amoxicillin-clavulanate, CRO: Ceftriaxone Sulbactam, GN: Gentamicin, IMP: Imipenem/Cilastatin, ACX: Ampiclox, LBC: Levofloxacin, NF: Nitrofurantoin, CXM: Cefuroxime, ZEM: Cefexime.
Figure 2. Percentage susceptibility test results for Pseudomonas spp.
![]()
Figure 3. Dectection of biofilm formation by Tube method.
![]()
Figure 4. Distribution of biofilm result for all the isolates tested.
deplorable state of the abattoir and poor sanitary practices employed in the slaughterhouse.
Results of antimicrobial susceptibility test using the multi-antibiotic disc were interpreted using the European committee on Antimicrobial Susceptibility Testing (EUCAST) 2022 standard breakpoints tables as shown on Table 3. E. coli showed 100% resistant to Cefexime, Cefuroxime, Ceftriazone, Sulbactam, Nitrofurantoin, Imipenem/Cilastatin, Amoxicillin-clavulanate and Ampiclox but was sensitive to levofloxacin, ofloxacin and gentamicin. The results of a study done by Gul et al., [12] showed similar results; E. coli was sensitive to Ofloxacin, Gentamicin and resistant to nitrofurantoin. Antibiotic resistance in E. coli is of particular concern because it is the most common Gram-negative pathogen in humans, multidrug-resistant strains and is easily transferable to other strains [15] .
![]()
Table 3. Antibiotics Sensitivity Test Result (inhibition zone diameter measured in mm).
KEY: OFX: Ofloxacin (5 μg), AUG: Amoxicillin-clavulanate (30 μg), CRO: Ceftriaxone Sulbactam (45 μg), GN: Gentamicin (10 μg), IMP: Imipenem/Cilastatin (10/10 ug), ACX: Ampiclox (10 ug), LBC: Levofloxacin (5 ug), NF: Nitrofurantoin (300 ug), CXM: Cefuroxime (30 ug), ZEM: Cefexime (5 ug). The result of antibiotics sensitivity above was interpreted using the European committee on Antimicrobial Susceptibility Testing Breakpoint tables for interpretation of MICs and zone diameters (EUCAST) 2022.
![]()
Table 4. Frequency biofilm formation.
Klebsiella spp. Klebsiella spp. was also sensitive to levofloxacin and ofloxacin but showed greater than 70% resistance to gentamicin, cefexime, cefuroxime, ceftriazone, sulbactam, nitrofurantoin, imipenem/cilastatin, amoxicillin-clavulanate and ampiclox. This result is similar to a study done by Makuvara & Marumure, [8] where amoxicillin-clavulanate and cephalosporins but showed 57.1% sensitivity rate to gentamicin. Another study by Odeniyi, [16] in Lafanwa abattoir in Ogun state, had variations in the isolates sensitive rates to cephalosporins and amoxicillin-clavulanate. Pseudomonas spp. was 100% resistant to all the drugs used in this study. A study conducted by Odeniyi, [16] did not agree with our findings because it presented an approximately 83% sensitivity to amoxicillin–clavulanate and cephalosporins. Pseudomonas spp. resistance to carbapenem and third generation cephalosporins is a real threat; the irrational and inappropriate use of antibiotics is usually responsible for the development of resistant strains of Pseudomonas spp. to antibiotics therapy [17] .
Proteus spp. showed 80% - 90% sensitivity to levofloxacin and ofloxacin while it had a 100% resistance to all the other antibiotics tested. This result is similar to a study conducted by Olawale et al., [18] where the isolates were 100% resistant to gentamicin, nitrofurantoin and cefexime. Another study by Lv et al., [19] presented approximately 70% resistance to imipenem and ofloxacin.
Biofilm producing bacteria are responsible for many recalcitrant infections and are difficult to eradicate. They exhibit resistance to antibiotics by various methods like efflux mechanisms, decreased growth rate and expression of resistance genes [20] .
In this study, as shown on Table 4, a total of 52 isolates were evaluated using tube method for screening of biofilm formation. The isolates formed 31 strong, 12 moderate and 9 weak biofilm formers respectively. Hassan et al. [21] in their study using the same method reported 21 strong, 33 moderate and 56 weak or non-biofilm producers. Figure 4 shows the distribution of biofils formed among the isolates tested. E. coli presented 25% strong formers while Proteus spp. a 100% weak biofilm forming isolates. Similar results were obtained in other related studies where the organisms presented strong biofilm forming activites [22] [23] .
The findings of this study confirmed the presence of some WHO priority organisms in Kwata meat market, which calls for concern as the meat serves many households and other commercial vendors. The presence of these organisms may be due to factors such as poor personal hygiene and sanitation procedures in the abattoir, inadequate surveillance and low education level of abattoir workers.
As a result of the high resistance pattern of the isolates to the antibiotics tested in the study, fluroquinolones, that is levofloxacin and ofloxacin should be the drugs of choice in treating microbial diseases or infections arising from meat in Kwata abattoir. Treatments of biofilm-associated diseases are harder to manage due to high resistance to conventional antibiotics even when complemented by host immune systems. This may lead to higher cost of infection treatment. The limitation of the study include our inability to carry out this study over an extended period of time and the identification of the organisms obtained from the samples collected was not done at the molecular level.
5. Conclusion
The high resistance pattern suggests the need for controlled use of antibiotics in animal feed as prophylaxis or to boost immunity will minimize antibiotic resistant trends and biofilm forming potentials of these organisms. The place of good personal and environmental hygiene can never be over emphasized, as it reduces infections and spread within any given community. It is also very important to have regular surveillance in this abattoir to regular unhygienic practices and appropriate sanctions meted out to defaulters.