Chemical Oxidation Effects on Anion Exchange and Nitrate Sorption Capacity of Biochar for Ruminal Methanogenesis Inhibition ()
1. Introduction
Methane (CH4) emission to the atmosphere is one of the main factors that cause climatic shifts. Currently, CH4 is considered the second most important greenhouse gas (GHG) after carbon dioxide (CO2) [1] . Researchers have estimated that CH4 is 25 times more global warming potential than CO2 [2] .
Ruminants play a crucial role in the global food chain; however, livestock is responsible for about 18% of global CH4 emissions [3] . Biochar is a charcoal-like substance that is produced by the pyrolysis of biomass under anaerobic conditions [4] . Many scientists actively discuss the potential of biochar to influence ruminal methanogenesis in ruminants, but details influencing its composition need further [5] .
Many different experiments have been conducted to test the efficacy of biochar in decreasing methanogens’ activity in the soil system. According to recent studies, biochar reduces CH4 emissions from paddy soils by 22% to 96% [6] . The methanogenesis processes in ruminants and soils are similar; however, research information on the effect of biochar on CH4 production in the rumen is scanty. In contrast, many more studies have been done on feeding nitrate (
) and its intermediate, nitrite (
), as alternate electron acceptors for dihydrogen to mitigate ruminal methanogenesis. Results are generally favorable [7] , but potential limitations in palatability or even potential
accumulation in blood still need to be addressed [8] . We hypothesized that nitrate would be associated with biochar and therefore prevent absorption of nitrate or
into the blood as long as preliminary studies document (
) sorption to biochar.
Some recent studies highlight the ability of biochar to absorb
and prevent nitrogen leaching from the soil system [9] . If biochar can absorb 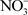 or
in the rumen, methanogens might be associated more closely with
in biochar’s matrix [10] for the latter to be more inhibitory to methanogens [11] . This way, biochar may provide direct interaction between
and methanogens, without letting the
to get absorbed into the blood system. Therefore, it is important to study biochar’s ability to absorb the compounds and exchange anions.
or
in the rumen, methanogens might be associated more closely with
in biochar’s matrix [10] for the latter to be more inhibitory to methanogens [11] . This way, biochar may provide direct interaction between
and methanogens, without letting the
to get absorbed into the blood system. Therefore, it is important to study biochar’s ability to absorb the compounds and exchange anions.
Some studies have discussed the efficacy of oxidized biochar in decreasing CH4 production [12] . A biochar with a positive redox potential is considered more chemically reactive; therefore, its sorption capacity was higher compared to a biochar with a negative redox potential [13] . Thus, different types of biochar may affect methanogenesis differently, and the association between the by-product redox potential and its electrochemical properties is a research priority. In the scope of the article, research experiments were established with the following objectives:
1) To determine the chemical composition of biochar in relation to feedstock and the pyrolysis parameters.
2) To estimate biochar’s
sorption capacity.
3) To analyze the anion exchange capacity (AEC) of biochar.
Based on the objectives, the following hypotheses were tested:
1) Chemical composition of biochar is diverse and associated with the chemical composition of biochar sources.
2) Oxidized biochar can absorb more
than untreated biochar.
3) Oxidized biochar has a higher AEC than untreated biochar.
In the article, analyses for determining the chemical composition, AEC, and
sorption capacity of biochar were completed.
2. Biochar’s Chemical Composition
The biochar used in the study is a product of hardwood pyrolysis, prepared in Watsonville, CA. The chemical composition of biochar and its basic characteristics were analyzed in Control Laboratories, Watsonville, CA. Basic physicochemical properties analysis results are presented in Table 1. The results of the chemical analysis are shown in Table 2.
According to Table 1, this source of biochar is rich in organic carbon and has an optimal ash content. About 50% of the hardwood chemical composition is taken by carbon [14] . Such characteristics of biochar as moisture content and bulk density, presented in Table 1, are similar to those for hardwood. For instance, the bulk density of hardwood can vary between 0.2 and 0.75 g/cm3, and the bulk density value of the analyzed biochar is in the same range [15] . The electrical conductivity (EC) of biochar is one of its most important characteristics because it determines the degree to which biochar participates in an electron flow under a specific environment. The EC values usually range from 2.5 × 10−4 to 399.7 S/m, which means that the EC value of the treatment used in the research is medium [16] . Small particle size indicates a high surface area of biochar [17] . The higher the surface area of biochar, the higher its ability to participate in redox reactions and, consequently, absorb
. In general, biochar has alkaline pH because of biomass pyrolysis [18] . The alkaline state of the by-product lowers the risk of dropping ruminal pH, which can prevent the occurrence of negative health conditions such as acidosis [19] .
According to Table 2, the major elements of the biochar’s chemical profile are sodium, iron, and manganese. All the elements actively participate in redox
![]()
Table 1. Basic physicochemical characteristics of biochar.
Note: ASTM stands for American Society for Testing and Materials.
![]()
Table 2. Chemical composition of biochar as determined by EPA 3050B/EPA6020 method.
Note: EPA stands for Environmental Protection Agency. ND* stands for “not detected” which means the result is below the reporting limit. “Declarations” means the EPA does not have a declared limit.
reactions, which, along with the treatment having a high surface area and medium EC, means that the by-product most likely will be actively involved in redox reactions in rumen fluid. The treatment also contains different heavy metals. However, none of them is present in concentrations that exceed their maximal safe levels. Thus, biochar is safe to be used during in vitro experiments.
3. Nitrate Sorption Capacity
The ability of biochar to absorb
can play a crucial role in reducing CH4 production in the rumen because nitrate’s storage in biochar particles would prevent
, produced by
reduction in the rumen, from getting into the blood system [8] . Instead,
would interact directly with the ruminal microbiota, and depressing methanogenesis [20] . Thus, it is important to assess biochar
sorption capacity.
Mao et al. (2008) observed that oxidized biochar is more chemically active and has a higher sorption capacity compared to an unoxidized one. Hence, oxidized biochar may be more efficient in
adsorption and, consequently, methanogenesis suppression in the rumen. In this study,
sorption capacity of oxidized, original, and reduced biochars was analyzed. Specifically, two oxidized treatments with different redox potentials were used to determine a possible trend in the relationship between biochar’s sorption capacity and its redox potential. One of the treatments was oxidized with hydrochloric acid (HCl), whereas distilled water (dH2O) was used as an oxidizing agent to prepare the second oxidized treatment. The procedure was adapted from Mao et al. (2008) with minor modifications [13] .
The procedure of biochar oxidation was divided into 5 main steps [13] :
Step 1. 5 M HCl/dH2O is added to biochar in the ratio of 1:6 (15 g biochar: 90 mL of 1 M HCl).
Step 2. The mix of 5 M HCl/dH2O and biochar was left overnight.
Step 3. Biochar was filtered through Whatman filter paper 2 with suction and rinsed with distilled water 4 times.
Step 4. Biochar was dried in a forced-air oven at 55˚C overnight.
Step 5. The redox potential of biochar oxidized with dH2O was measured to confirm that biochar was oxidized.
The procedure of biochar reduction hasn’t been extensively described in the existing literature. Therefore, in the scope of the research, we decided to use the same methodology as was used to oxidize biochar, except for replacing the oxidizing agent with a reducing agent. The procedure of biochar reduction was also divided into 5 main steps:
Step 1. Reducing solution (3.125 g L-Cys HCl·H2O, 20 mL 1 M NaOH, 3.125 g Na2S·9H2O, 475 mL reduced distilled H2O) is added to biochar in the ratio of 1:6 (15 g biochar: 90 mL of reducing solution).
Step 2. The mix of reducing solution and biochar was left overnight.
Step 3. Biochar was filtered through Whatman filter paper 2 and rinsed with distilled water 4 times.
Step 4. Biochar was dried in the oven at 55˚C overnight.
Step 5. The redox potential of biochar was measured to make sure that the value was decreased and confirm that biochar was reduced.
After oxidation, some of the biochar treatments were left in a package and stored for a month. After one month, the redox potential of both biochars was measured to determine if redox changed (Table 3).
Oxidized biochar redox potential tends to increase with time. Therefore, oxidized biochar is a chemically unstable compound. The increase in redox potential values can be explained by the exposure of the treatment in the packages to
![]()
Table 3. Changes in redox potential of oxidized biochar before and after 1 month of storage.
the oxygen in the air. Thus, oxidized biochar must be used as soon as possible after the treatment is prepared.
After preparing the oxidized treatments and measuring their redox potential, samples for
sorption capacity analysis were prepared following the procedure described by Jatana et al. (2020) with minor changes made [21] :
Step 1. The optimal ratio of biochar to
is 2:1.5 as has been determined based on a preliminary study conducted in Firkins’ Lab, The Ohio State University, Columbus, OH. 0.5 g of biochar was mixed with NaNO3 according to the ratio.
Step 2. 0.5 g of pure biochar treatments were extracted with 35 mL of 1 M KCl solution to prepare samples for evaluating the content of
in the treatments themselves. Samples were prepared in duplicates.
Step 3. The solution of biochar and 1 M KCl was placed on a rotary shaker for 2 hr at room temperature.
Step 4. The solutions were centrifuged at 1315 × g for 8 min.
Step 5. The supernatants were filtered through the Whatman filter paper 2.
Step 6. The filtrate solutions are analyzed for
in a nutrient analyzer [22] .
The results of the test are presented in Table 4.
The data in Table 4 indicates every treatment’s ability to recover
, which highlights the potential ability of biochar to store these anions. The original biochar had the highest potential to adsorb
As opposed to the related studies’ data, oxidized biochar had the lowest
sorption capacity. The biochar, which was oxidized with HCl, absorbed less
than the one that was oxidized with distilled water, even though it had a higher redox potential. This
![]()
Table 4. Means for nitrate recovered in fractions as influenced by biochar oxidation state.
Note: Original
content in solution is 66.3 mg (per 35 ml).
in filtrand values refer to the amount of
in the chemical profiles of the biochar in different oxidation states. The ± values refer to standard errors.
-recovered values were calculated using the following formula: Original
content in the solution −
in the filtrand −
in the filtrate. a,b,cMeans in the same row with unlike superscripts differ (P ≤ 0.05).
response can be explained by referring to the chemistry of NaNO3. This salt reacts with concentrated acids and does not react with diluted ones [23] . The acid that was used to oxidize biochar was a diluted one. Thus, there apparently was limited ion exchange between the oxidized treatment and NaNO3. This underlines the necessity of using only certain chemicals as oxidizing agents because even if the agent increases the redox potential of the by-product, it does not necessarily make biochar more chemically active in relation to
.
The biochar oxidized with dH2O was also not able to adsorb more
than the original biochar (Table 4). This could happen because cations that form bridges with
, such as sodium, were rinsed out from the biochar surface during filtration [24] . The dH2O could wash some chemically active ions out from the biochar surface area or decrease the content of the cations in biochar particles. This could lead to a lesser chemical reactivity of biochar and, as a result, lesser
absorption.
According to the results of statistical analysis performed using the ANOVA test ran on the basis on R Studio 2023.03.1-446 having a randomized complete block with two runs that were modeled as random effect, the treatments were different according to a protected least significant difference test in recovering
(Figure 1). The change in
absorption values between the treatment groups is notable [25] . Biochar oxidized with HCl was not significantly different from both oxidized using dH2O and reduced treatments. Based on the statistical analysis, changes in biochar’s redox potential can significantly alter its ability to retain
.
According to the results of the experiment, biochar oxidation does not guarantee high
binding. There was no association between
sorption capacity and the redox potential of biochar. However, the
sorption capacity of oxidized biochar can be changed depending on what chemical compound
![]()
Figure 1. Means for sorption of
to original biochar or biochar that was previously oxidized (distilled water or 5 M HCl) or reduced (reducing solution). Letters (a, b, c) differ (P ≤ 0.05), and the bars represent standard errors.
is used as an oxidizing or reducing agent [26] .
4. Biochar Anion Exchange Capacity
The AEC refers to the total positive charge of a material. For the study, the AEC estimates biochar’s ability to attract and absorb different anions. AEC was measured for oxidized, reduced, and original biochar treatments. Chloride (Cl−) served as a marker anion for evaluating biochar’s AEC. The procedure for AEC test sample preparation is divided into 8 steps [27] :
Step 1. 50 g of biochar was dissolved in deionized water.
Step 2. This mixture was shaken on a reciprocating shaker for 24 hr, after which, the slurries were transferred to dialysis tubing (Spectra/POR® MW6-8000, 32 mm).
Step 3. 2 mL of 1 M KCl was added to the suspension, shaken for 2 days, then rinsed through a 0.45-μm Teflon filter.
Step 4. Biochar was combined with 2 mL of 2.5 M CaCl2 and 50 mL of water.
Step 5. Samples were returned to the shaker for 2 days.
Step 6. Biochar slurries were subsequently diluted to 200 mL in a volumetric flask.
Step 7. A portion of step 6 was filtered through an IC Acrodisk® 25-mm syringe filter with a 0.45-μm Supor® PES membrane, and 10.0 mL of filtrate was diluted to 100 mL.
Step 8. The solution was analyzed for Cl− anions content using ion-exchange chromatography.
The results of the test are presented in Table 5.
As shown in Table 5, means were very precise such that all treatments were different from each other. Differing batches of biochar might have higher standard errors and fewer treatment differences. However, in this study, reduced biochar was able to adsorb more anions than other treatments; consequently, its AEC is the highest among the treatments. The lowest AEC was observed in the
![]()
Table 5. Means for Cl− absorbed recovered in fractions as influences by biochar oxidation state.
Note: The original solution contained 3.42 g of Cl− (per 52 ml). Anion exchange capacity of the treatments was estimated based on the ability of biochar to recover Cl−, which was used as a marker anion. The ± values refer to standard errors. a,b,c,dMeans in the same row with unlike superscripts differ (P ≤ 0.05).
case of original biochar, whereas the by-product oxidized with dH2O retained less than the one oxidized with HCl but more than the original one. Thus, according to the cases of original biochar and both oxidized treatments, there is an association between redox potential and AEC because the higher the redox potential, the higher the AEC value [27] . However, the results obtained from the reduced treatment analysis do not fit the pattern. Such deviation can be explained by the biochar being reduced using a solution that contains L-cysteine. L-cystein is much more reactive than the oxidizing agents used to increase biochar’s redox potential in the scope of the study. The high chemical reactivity of L-cystein occurs due to the presence of sulfur-based, amine, and carboxyl functional groups in the structure of the compound [28] . Chemical composition of a material serves as an AEC-forming factor and has a higher influence on the ability to absorb anions than redox potential [29] . Therefore, it should be highlighted that AEC depends on different factors, some of which influence the characteristic stronger than others [30] .
In addition, according to the results of ANOVA test ran on the basis on R Studio 2023.03.1-446 having a randomized complete block with two runs that were modeled as random effects, and least significant difference test, there is a noteworthy difference in absorbing Cl− between the treatments (Figure 2). As explained previously, there is high precision and causing all means to be different statistically but perhaps not functionally [25] . Nevertheless, it should be underlined that all treatments showed high ability to recover Cl−.
The AEC values using Cl− were not associated with
adsorption, even though AEC characterizes the ability of different materials to attract different anions, including
[31] . However, the findings point out that biochar interacts with different anions differently. For instance, the original biochar absorbed more
than any other treatment but did not succeed in absorbing
![]()
Figure 2. Means for sorption of Cl− absorption to original biochar or biochar that was previously oxidized (distilled water or 5N HCl) or reduced (reducing solution). Different letters (a, b, c, d) differ (P ≤ 0.05). Standard error bars are not visible because their value is too small.
Cl−. This trend can be partially explained by considering the treatments’ pH. The AEC increases with decreasing pH [24] . The original treatment has the highest pH (10.3) and, therefore, the lowest AEC. The biochar that was oxidized with dH2O had a pH (9.5) lower than the original treatment but higher than the biochar oxidized with HCl (1.3); consequently, its AEC is higher than the original biochar’s but lower than the sample oxidized with dH2O. Reduced biochar, however, has the highest AEC and an alkaline pH (9.8). The reason for this may be that the by-product was reduced with a solution that includes L-cysteine, which, as has been mentioned before, contains 3 highly reactive functional groups, which may give it the ability to react and attract anions more intensively [28] .
It is important to notice the difference between Cl− and
retention by the same treatments. The original and treated biochars were able to retain much more Cl− than
. It can be partially explained by a higher chemical activity and strength of Cl− compared
. In addition, for AEC analysis, samples were mixed with CaCl2. Under the conditions of anaerobiosis, quinones on biochar surface form pyrroloquinoline quinone (PQQ) by comproportion between quinone and quinol forms. Ca2+ binds to PQQ, forming a stable complex [32] . Since quinones are major compounds in the chemical profile of biochar and are reactive, it is possible that Ca2+ ability to bind to PQQ allowed the treatments to absorb more Cl− than
. Na+, however, can also bind to PQQ but the reaction rate in this case is much lower [32] . Therefore, there is a significant difference between biochar’s ability to absorb
and Cl−.
In conclusion, there is a direct association between the redox potential of the treatments and their AEC. However, redox potential can lose its influence on biochar’s AEC in the presence of organic compounds that have strong functional groups in their structure on biochar’s surface. It should also be noted that AEC of biochar and its ability to absorb certain anions such as
are not necessarily linked.
5. Conclusions
Based on the results presented above, the following conclusions were made:
1) The original hypothesis related to biochar’s chemical composition has been supported. The chemical profile of biochar consists of different chemical elements, including heavy metals, and is related to the chemical composition of the biochar source, hardwood.
2) The hypothesis that stated that oxidized biochar recovers more
has been refuted. The chemical oxidation of biochar did not enhance the ability of the by-product to absorb
. In fact, the capacity was lowered by oxidation.
3) The hypothesis, which assumed that oxidized biochar has a higher AEC, has been partially proven. However, even if the redox is high, the chemical profile of biochar’s surface plays a more crucial role in determining the ability of the by-product to exchange anions with the environment. In the case of the study, we emphasize that compounds, containing highly reactive functional groups, can dominate in their effect on AEC of biochar over redox potential.
The results of the study can be used for predicting biochar’s redox potential influence on ruminal methanogenesis. Moreover, the research supports further study on the connection between electrochemical properties among different biochar lots and their relative abilities to interact with weak anions such as
in future CH4 abatement studies.
Acknowledgements
Dr. Murray Minnema, Control Laboratories, Watsonville, CA.