1. Introduction
Diabetes are metabolic diseases that have become a public health issue due to the alarming and increasing number of cases worldwide and in Africa in particular. It is a pandemic disease that is expanding at an alarming rate. Recently, it has been reported that 422 million people worldwide have diabetes and this figure is growing and expected to reach 629 million by 2045 [1] [2] [3] . This is the ration of one in ten people and more importantly, half of those diabetes cases are still undiagnosed. The disease is characterized by the severity of its complications and this is the leading cause of end-stage renal failure, hypertension, blindness, lower limb amputations and this will be the 7th leading cause of death by 2030 [3] . Treatments of diabetes have long been restricted to dietary changes, insulin injections and oral antidiabetic drugs uptakes [4] . The requirements to access a modern treatment are constrained by the higher cost and safety of medications as well as modern infrastructures accessibility and qualified personnel, these therefore lead African populations to turn to traditional medicine [5] . This is as a result to a serious problem caused by side effect and high cost of manufactured medicines [6] . Medicinal plants constitute a medical potential that is accessible, available and of lower cost [5] . Several plants have already proven their effectiveness and efficiency in local pharmacopeia and molecules associated with anti-diabetic activities have been isolated, unfortunately, many of these plants do not have scientific data available, on scientific basis to support their efficacy and safety [7] . On this perspective, we undertook the study on the effect of aqueous extract of Tetracera potatoria (Dilleniaceae), a plant of the Congolese pharmacopoeia, used on mellitus diabetes and the prevention. Its complications in the Wistar rat to compensate the limitations of modern medicines in the treatment diabetes and glycaemia, due to side effects and higher cost.
2. Material and Methods
Plant leaves material have been used in this study to access their mellitus diabetes effect on Wistar rat.
2.1. Material
Plant and animal materials were used in this study.
2.1.1. Plant Material
In this study, plant leaves material of Tetracera potatoria were collected in the field in September 2021 in Mossendjo in the Niari Department (Republic of Congo). The identification was then confirmed by botanist scientists at the Institut de Recherche en Sciences Exactes et Naturelles (IRSEN) of Brazzaville by comparing with the Catalogue des Plants Vasculaires du Congo of May 1988, SITA and MOUTSAMBOTE [8] . These leaves were air-dried at room temperature for two weeks, pulverized and thereafter subjected to a decoction, as previously described [9] .
2.1.2. Animal Material
Male rats (Wistar strain) with a body weight ranging between 250 and 300 g were used for this experiment. Animals were raised at the animal house of the Faculty of Sciences and Techniques of the University Marien NGOUABI. And were then maintained under standard conditions alternating 12 hours under light and 12 hours of darkness exposure, at room temperature, with free access to food and water [10] . There was no animal sacrificed in this experiment and animal handling was done following the strict animal handing guideline available.
2.2. Methods
2.2.1. Preparation of the Aqueous Extract
For the preparation of aqueous extract, 100 g of Tetracera potatoria leaf powder were mixed with 1000 ml of distilled water and boiled for 15 minutes. After cooling down and filtration, the decoction obtained was evaporated at 65˚C using a Bucchi rotary evaporator as previously described [11] .
2.2.2. Diabetes Induction
Mellitus diabetes similar to type II diabetes was induced in mice by intraperitoneal injection of a single dose of Alloxan (ALX, SIGMA) at 60 mg/kg body weight to male rats weighing between 250 and 300 g, after 14 - 16 hours fasting. At 72 hours after diabetes induction, blood sugar levels were taken from the main tail vein using a code free glucose meter [12] and the fasting insulin levels was recorded. Rats with high blood glucose levels greater than or equal to 1.35 g/L were considered as diabetic.
2.2.3. Evaluation of Hypoglycaemic Activity
Normoglycemic male rats subjected to a 16-hours fasting before being tested were divided into four (4) batches of five rats each and treated as follow. Batch 1 used as a negative control, received 10 mL/Kg of distilled water (DW) per os and batch 2 used as positive control, was treated with 5 mg/Kg of Glibenclamide per os as a reference product. Whereas batches 3 and 4 received respectively 200 and 400 mg/Kg of the aqueous extract of Tetracera potatoria per os. Glycosylated blood was punctuated at the beginning of the experiment and after 5 hours of incubation, glycaemic blood was taken after every hour.
2.2.4. Evaluation of Antihy-Perglycaemic Activity
This activity was evaluated by inducing hyperglycaemia by oral route of 10% glucose in normoglycemic rats at 3 g/kg. These animals were treated and divided into four (4) other batches of five (5) rats each and treated as follows. Batch 1 (negative control) received distilled water at 10 mL/Kg; Batch 2 (positive control) received Glibenclamide at 5 mg/Kg and batch 3, 4,5 and 6 received respective doses of 50, 100, 200 and 400 mg/Kg of Tetracera potatoria leaf decoction, respectively. One hour after water administration of glibenclamide followed by four doses of the extract, animals were overload with glucose. The blood glucose (glycaemia) measurements were taken respectively after 30 minutes, one hour, two and three hours after the glucose intake.
2.2.5. Evaluation of the Anti-Diabetic Activity of the Aqueous Extract of T. Potatoria Leaves
1) Glycaemia monitoring of diabetic rats
The diabetic rats subjected to a 16 hour fasting, were divided into four (4) batches of five (5) rats each and treated as follows: batch 1 (negative control): the rats received distilled water (10 ml/kg); batch 2 (positive control): the rats received Glibenclamide at a dose of 5 mg/Kg; batch 3 and 4 or test batches: the rats received the doses of 200 and 400 mg/Kg of the aqueous extract T. potatoria respectively. At the beginning of the experiment, baseline blood glucose levels were taken, and then blood glucose monitoring was done every hour and extended for 5 h.
2.3. Sub-Acute Treatment of Diabetic Rats
Normal and diabetic rats selected for this treatment were first fasted for 16 hours then divided into five (5) batches of six (6) rats each. The animals were treated daily at the same time for 28 days as follows: Batch 1 of normal animals receiving distilled water (DW) at 10 ml/Kg; Batch 2 of diabetic animals receiving distilled water (10 ml/kg); Batch 3 of diabetic animals receiving Glibenclamide at 5 mg/Kg; Batch 4 and 5 of diabetic animals receiving 200 and 400 mg/Kg of T. potatoria aqueous extract, respectively.
For each animal, weight measurement was performed daily and blood glucose was taken only at the end of each week after 16 h. Twenty for hours (24 h) after the last gavage, animals were anesthetized by exposure to diethyl ether vapors. Blood was then collected from the eyes by puncture in the retro-orbital sinus and put into the dry EDTA tubes [12] . After centrifugation of the blood at 3000 rpm for 15 minutes, the serum was recovered and used for biochemical assays, and for the determination of glycated haemoglobin. The biochemical assay was performed using enzymatic colorimetric method in serum (Cypress kit, Spain).
2.4. Phytochemical Study
The identification of the chemical families was done by tube reactions which allow, from tests with different reagents; to access chemical secondary metabolites groups present in a plant extract [13] .
2.5. Data Analysis
The chemical screening and Biochemical data analysis was performed using excels spread sheet. Results were expressed as mean ± standard errors and the variance was studied by Student’s t-test. The significance level was set at p < 0.05.
3. Results
Effects of aqueous extract of T. potatoria leaves on blood glucose levels in normal glycaemia induced rats were accessed using the following tests
3.1. Hypoglycaemic Effect of the Aqueous Extract of T. potatoria Leaves (Table 1)
The administration of aqueous extract per os at doses of 200 and 400 mg/Kg and glibenclamide at 5 mg/kg (Table 1), results in a significant (p < 0.01) reduction of baseline mean blood glucose levels from the first hour, with respective percentages of blood glucose reduction of 10.96%, 16.00% and 12.17% versus 4.22% for controls given distilled water. In all rats treated with glibenclamide and aqueous extract at doses of 200 and 400 mg/Kg, a more significant reduction (p < 0.001) was observed up to the 5th hour with respective percentages of blood glucose reduction of 30.43%, 25.86% and 55.45% versus 7.46% for distilled water.
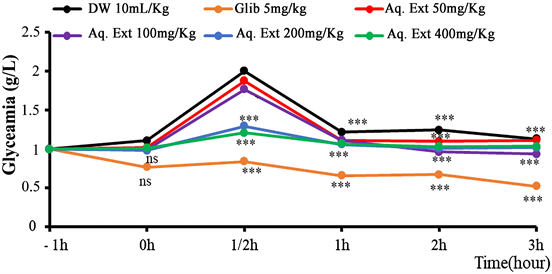
3.2. Effect of the Aqueous Extract of the Leaves of T. potatoria on the Blood Glucose Levels of Normal Rats Subjected to the Orally Induced Hyperglycaemia Test (Figure 1)
The administration of different doses of the extract and glibenclamide did not reduce the mean blood glucose levels of the rats after one hour, except the effective effect of glibenclamide, a pure manufactured product. After 30 minutes of glucose intake (3 g/Kg), a significant increase (p < 0.001) in mean blood glucose
![]()
Table 1. Evolution of Mean blood sugar levels in normal rats as a function of extract.
DW: distilled water; Aq Ext: aqueous extract; Glib: Glibenclamide; Significant difference from the control lot treated with distilled water: *p < 0.05; **p < 0.01; ***p < 0.001; the effect extract ns on the evolution of blood sugar: ns not significant.
levels with peaks around 2 g/l was observed. Rats treated with distilled water show the greatest peak, above 2 g/l, compared to the others. Doses of 200 and 400 mg/kg appear to be more effective, compared to the two others of the extract, because their peak blood glucose levels approach the normal blood glucose level and are close to glibenclamide. It is observed that three (3) hours after glucose overload, all rats tend to regulate their blood glucose levels (Figure 1).
3.3. Evaluation of the Anti-Diabetic Effect of the Aqueous Extract of the Leaves of T. potatoria (Table 2)
We observed that the extract is active on the glycaemia of diabetic rats (Table 2), indeed, the doses of 200 and 400 mg/Kg of the extract, appearing more effective
DW: distilled water; aq Ext: aqueous extract; Glib: Glibenclamide; Significant difference from the control lot treated with distilled water: *p < 0.05; **p < 0.01; ***p < 0.001; ns: not significant and did not make any changes in blood glucose levels in normal and diabetic rats treated for four weeks.
Figure 1. Changes in blood glucose levels in normal and diabetic rats treated for four weeks [10] .
![]()
Table 2. Evolution of mean blood glucose levels in diabetic rats after treatment with aqueous extract of T. potatori [10] .
AE: Aqueous extract; Glib: Glibenclamide; *p < 0.05; **p < 0.01; ***p < 0.001: Significant difference from the control batch treated with distilled water; ns: not significant and did not make any change in blood glucose levels in diabetic rats after treatment with aqueous extract of T. Potatoria.
during the orally induced hyperglycaemia test, significantly reduce the blood glucose levels of rats from the first hour for the 200 mg/kg dose (p < 0.05), from the second hour for the 400 mg/kg dose (p < 0.01), compared to diabetic rats given water which show no reduction in blood glucose levels until the 5th hour. Similar to glibenclamide reducing blood glucose from the first hour, both doses of the extract become significantly more active (p < 0.001) at the fifth hour with respective blood glucose reduction percentages of 41.12%, 39.97%, 27.8% versus −5.19% for distilled water. The 200 mg/kg dose appeared to be more active than the 400 mg/kg dose from the first hour to the fifth hour.
3.4. Glycaemic Monitoring of Diabetic Rats after Treatment
3.4.1. Evolution of Blood Sugar Level (Figure 2)
The daily administration of the 200 and 400 mg/kg doses of the extract and glibenclamide allows the very significant (p < 0.001) decrease of the blood glucose level from the first one compared to the diabetic rats that received only distilled water).
All rats treated with both doses of the extract survived until the fourth week, all showing a trend towards normalization of blood glucose; whereas diabetic rats receiving only distilled water all showed significant (p < 0.01) increases in blood glucose compared to treated rats and all died after two weeks (Figure 2).
3.4.2. Effect of the Evolution on Weight-Gain (Figure 3)
The weight gain started at week 3 and Figure 3 shows that both normal rats and diabetic rats treated with glibenclamide, aqueous extract doses increase significantly weight gain (p < 0.001); while diabetic rats given only water loss weight gradually and die 2 weeks acute during 4 weeks (Figure 3).
3.4.3. Effect of Aqueous Extract on Biochemical Parameters (Table 3)
The aqueous extract at the dose of 400 mg/kg prevents the occurrence of metabolic complications (Table 3), as the markers of creatinemia, transaminases,
![]() *p < 0.05; **p < 0.01; ***p < 0.001; ns: not significant.
*p < 0.05; **p < 0.01; ***p < 0.001; ns: not significant.
Figure 2. Changes in blood glucose levels in normal and diabetic rats treated for four weeks.
![]()
Figure 3. Evolution of boby weight of normal and diabetic rats subjected to subcutaneous treatment for four weeks.
![]()
Table 3. Biochemical parameters of rats treated for 28 days.
Results are expressed as mean ± standard error (MSE), n = 5 rats per batch. ns: non-significant difference; *p < 0.05; **p < 0.01; ***p < 0.001; significant difference compared with control rats treated with distilled water (DW). DW: distilled water; AE: Aqueous Extract; CREAT: creatinin; ASAT: aspartate amino transferase; ALAT: alanine amino transferase; TG: triglyceride; TC: total cholesterol; HDL: high-density lipoprotein; LDL: low-density lipoprotein; HbA1c: glycated haemoglobin, Glib: Glibenclamide.
lipid profile and glycated haemoglobin do not vary significantly, compared to normal rats. At the corresponding dose of 200 mg/kg, there was a significant increase in ALT compared to normal rats; while all other biochemical parameters remained normal.
Diabetic rats treated with glibenclamide at 5 mg/kg show an increase in all transaminases (ALAT and ASAT) compared to normal rats; while all other parameters remain normal.
3.5. Phytochemical Profile of Tetracera potatoria Leaves
Different tubes reactions made with the aqueous extract of the leaves of Tetracera potatoria show chemical screening profile (Table 4). The following secondary metabolites were detected: tannins, flavonoids, sterols, terpenoids, mucilage, anthraquinones and carbohydrates in small.
Summary
Our results show that Tetracera potatoria leaves are able to protect normal rats against hyperglycemia, lower blood sugar levels and prevent complications in diabetic rats. This justifies the use of its leaves in traditional medicine in the Republic of Congo.
4. Discussion
The rats chemically challenged orally with glucose through peripheral tissues for high blood sugar induction showed induced hyperglycaemia. These hyperglycaemic mice were further treated with aqueous extract of Tetracera potatoria. The phytochemical analysis of Tetracera potatoria extract show that the bioactive substances with hypoglycaemic and antidiabetic properties. These properties are also able to protect animals against the occurrence of hyperglycaemia, demonstrating the protective effect, already demonstrated by other researchers with other medicinal plant species extracts such as Trilepisuim madagascariense D.C. Leuwenberg [14] , Persea americana [15] . These results obtained following the administration of the aqueous leaves extract of the Tetracera potatoria to normoglycaemic and diabetic rats, resulted in a significant decrease of the average blood glucose levels, compared to normal rats and diabetic rats that received only water as previously demonstrated on the aqueous extract of Trilepisuim madagascariense D.C. Leuwenberg leaves [14] .
It is also noted that aqueous extract of the leaves of Tetracera potatoria
![]()
Table 4. Secondary metabolites present in leaves of Tetracera potatoria.
Significance of symbols: Low presence “+”; Moderate “++”; High presence “+++”; Absence “−”.
activity varies according to the doses administered and it is therefore deduced from this observation, the extract-dose-dependent effect. The same fact was also observed with Bridelia ferruginea extracts [16] and Icacina senegalensis [17] .
We observed that the administration of the aqueous extract at doses of 200 and 400 mg/Kg to diabetic rats resulted in a highly significant (p < 0.001) reduction in mean blood glucose levels in all diabetic rats compared to the mean blood glucose levels in the baseline, 5 hours later. This reduction is similar to that of diabetic rats treated with glibenclamide, compared to diabetic rats that received only distilled water. We can therefore deduce that in addition to the hypoglycaemic and anti-hyperglycemic effects, this extract has an anti-diabetic activity [15] .
All effects of aqueous extract of Tetracera potatoria leaves can be attributed to the action of flavonoids that act by improving the sensitivity of the body’s cells to insulin [18] . Indeed, the antihyperglycaemic power of flavonoids has been reported by other researchers on the extracts of Persea americana [15] , Zizyphus mauritiana [19] , Bridelia ferruginea [16] . This was also observed on Citrus aurantifolia [20] and has also shown that flavonoids could have antihyperglycemic properties by acting on the enzymatic activity involved in the hepatic metabolism of glucose by activating glycolysis pathway and neoglucogenesis inhibition [21] . It has also been shown that flavonoids can increase glucotransporter 4 (GLUT4) activities in adipocytes for glycogen synthesis in the liver [20] .
The same treatment of diabetic rats with this aqueous extract at same doses at 5 mg/kg bw for four weeks, respectively, induced a significant decrease in mean blood glucose levels with percentages of blood glucose reduction, respectively, of 55.56%, 63.34% and 62.38% compared to healthy control batches. This observation is similar to the effects of several plants used in traditional pharmacopoeia in Cucurbitacea and Anacardiaceae such as Momordica charantia L. [22] , Anacardium occidentale [23] , respectively, tested on blood glucose levels of diabetic rats. Indeed, it has been demonstrated that the aqueous extract of the fresh fruits of Momordica charantia L., an anti-diabetic plant [24] , leads to a decrease in blood sugar levels in mice with induced diabet by the action of alloxan at a dose of 200 mg/kg [22] .
This finding has been also evidenced in diabetically induced rats by the action of streptozotocin on rats made diabetic in the presence of ethanolic extract of Anacardium occidentalis leaves [23] . This was similarly demonstrated in antihy-perglycaemic effect of aqueous extract of Ageratum conyzoides on rats made diabetic by streptozotocin effects [25] . Consistently, this effect was similarly observed with a methanolic extracts on rats made diabetic by streptozotocin [26] .
The aqueous extract of Tetracera potatoria could be acting using a similar mechanism of action or other, to reduce the glycaemia of diabetic rats. Indeed, glibenclamide binds to its receptors on the surface of the pancreatic beta cell membrane to depolarize the membrane followed by the opening of calcium-dependent calcium channels, which leads to calcium entry into the cell. This of calcium entry will result in the release of insulin which will induce a decrease in blood glucose levels [27] .
In our study, it is showed that the injection of Alloxan induces diabetes, which is characterized by a body weight loss in the control group of diabetic rats. This decrease is about 96% compared to the initial body weight after two weeks of treatment with distilled water. Whereas, the healthy control group is still on a steady increase of 104.58% during the same period. This finding reflects the effects of the injection of streptozotocin in male Wistar albino rats which caused a significant decrease in body weight within three weeks [27] . This animal’s body weight loss is probably due to insulin deficiency which leads to a decrease of amino acids intake by the tissues which consequently leads to the reduction in protein synthesis [28] . In addition, many studies suggest that the loss of body weight in diabetic rats can be explained by an increase in lipid and protein catabolism due to carbohydrate deficiency [28] . In the treated diabetic group of mice, the daily gavage administration of the aqueous extract at doses of 200 and 400 mg/kg for four weeks improved the change in body weight compared to the healthy control group. In this group there was an increase of 110.9%, 107.67% and 109.31% respectively after four weeks of treatment compared to the initial weight of the rats. The ability of the extract to protect diabetic rats from massive loss of body weight seems to be due to 1) its ability to reduce lipid levels, 2) to its hypoglycaemic effect [29] [30] and thus 3) to its ability to reverse gluconeogenesis action and control protein loss [31] [32] .
The creatinin hormone is known as an excellent marker of renal function; indeed, its increase leads to renal dysfunction. A significant reduction in creatinin levels in diabetic rats treated with the aqueous extract of Tetracera potatoria was noted for 4 weeks later. This aqueous extract has improved renal changes in diabetic rats, or it decreases the catabolism of creatinin and phosphocreatine in the muscles, this phenomenon has been previously demonstrated [33] .
Alanine aminotransferase (AAT) is a liver-specific enzyme, making it an important and highly sensitive indicator of hepatotoxicity [34] . Aspartate aminotransferase is also an indicator of hepatocyte destruction although it is also found in the heart, skeletal muscle; lung and in the kidney [35] . Levels of both enzymes increasingly rise when the liver is damaged for a variety of reasons including hepatic cell necrosis, hepatitis, cirrhosis as well as hepatotoxicity of certain drugs [36] . In this study, a highly significant (p < 0.001) increase in the concentration of these two enzymes in diabetic rats treated with glibenclamide found, compared to healthy control rats; on the other hand with 200 mg/kg extract shows a significant increase (p < 0.05) mainly in ALAT and not significant for ASAT. The 400 mg/kg dose shows no significant increase in either liver enzyme.
Lipids play an important role in the pathogenesis mechanism of mellitus diabetes. Hypercholesterolemia and hypertriglyceridemia are observed in the pathology of diabetes and are risk factors for atherosclerosis and coronary heart disease [37] . It has been reported that elevated rate of lipids serum in rats made diabetic by streptozotocin play an important role in the pathology of diabetes [38] .
In this study, we found that in diabetic rats treated for four weeks with the aqueous extract of T. potatoria at respective doses of 200 and 400 mg/kg showed a decrease in serum total cholesterol concentration and serum triglyceride concentration compared to the healthy control group (Table 3) for total cholesterol and triglyceride. The antihypercholesterolemic effect of several bioactive molecules such as flavonoids, triterpenes and saponins has been reported [39] . Furthermore, the lipid-lowering effect of this extract can be attributed to flavonoids and triterpen actions. Consequently, Glycated haemoglobin is known to be an important marker in diabetes control and its level in diabetic rats treated with aqueous extract of T. potatoria did not change their status significantly compared to the healthy control rats whereas diabetic rats treated with glibenclamide showed a non-significant increase compared to healthy control rats. Likewise, Flavonoids with antidiabetic activity have been identified in the extracts of some plants [14] [40] and [41] . In addition, other chemical substances contained in the leaves extract of T. potatoria such as tannins, anthraquinones, terpenoids could also be responsible for this putative activity. These chemical families are very effective in the treatment of degenerative diseases, including diabetes [42] [20] . This activity may also be combined action of these substances which coerce with other substances such as flavonoids and terpenoids; this coercive action as has been observed with the leaves extract of Gnidia glauca Lin [21] and Lycium shawil [43] .
5. Conclusion
Tetracera potatoria leaves extract contains chemical substances with hypoglycaemic and antidiabetic properties whose activities may affect singularly or in coercive manner the hyperglycaemia in chemically challenged and on normoglycemic rats. Compared to control rats, the extract shows a significant decrease of average blood sugar levels. The extract contains a bioactive substance which implies the decrease of glycaemia in diabetic rats. Biochemical profile of the extract following their action in the treatment of the diabetic rats with the aqueous extract could justify the use of T. potatoria in traditional medicine for the treatment of diabetes in traditional pharmacopeia could be a way forward to an alternative medicine in diabetes treatment in least developed countries.
Acknowledgements
We would like to express our special thanks to traditional herbalists from Mossedjo for their friendly collaboration. Our gratitude to the laboratory of de Pharmacodynamics and Physiopathology team for experience sharing, and for supplying us with Wistar rats used for our experiments.
Author Agreement
All authors of the manuscript have read and agreed to its content and are accountable for all aspects of the accuracy and integrity of the manuscript.
This is our original work and it has not been considered or reviewed by any other publication and has not been published elsewhere in the same or a similar form.