Diabetes Ketoacidosis in Pregnancy: A Retrospective Study from the Teaching Hospital of Pikine ()
1. Introduction
Although rare, diabetic ketoacidosis (DKA) during pregnancy constitutes a medico-obstetric emergency which involves both maternal vital prognosis and that of the foetus [1] . In pregnant women this metabolic complication is defined by the biochemical triad of hyperglycaemia > 2 g/L, ketonemia ≥ 3 mmol/L, and high anion gap metabolic acidosis with pH < 7.30 [2] . This metabolic disorder usually occurs during the second and third trimester due to increased insulin resistance. DKA is most often encountered in cases of type 1 diabetes mellitus (T1DM) and sometimes coincides with diabetes onset, but it is also reported in women with type 2 diabetes mellitus (T2DM) or gestational diabetes mellitus (GDM) [3] . About 3% of diabetic pregnancies have DKA, and the incidence rate is higher with poorly controlled T1DM. Maternal complications include acute kidney injury, adult respiratory distress syndrome, myocardial ischaemia, and cerebral oedema with mortality rates of <1%. Foetal complications include abnormal heart rate, a high risk of preterm birth, hypoxia, acidosis, and markedly elevated mortality rates that range from 10% to 35% [1] [4] . DKA in pregnancy can be precipitated by infections, newly diagnosed diabetes, intractable vomiting (hyperemesis gravidarum), starvation, inadequate doses of insulin, non-adherence to insulin therapy, and medications such as beta-sympathomimetic tocolytic therapy and glucocorticoid therapy prescribed to enhance foetal lung maturity [5] .
Data on the occurrence of DKA in pregnant women in Africa is rare [6] and no Senegalese studies were identified. The objectives of this study were to evaluate the epidemiological, diagnostic, and prognostic characteristics of DKA in pregnant women in Dakar hospitals and to study the predictive factors of its occurrence. It is in this context that a retrospective study was conducted over an 8-year period at the National University Hospital Centre in Pikine for pregnant women with DKA.
2. Methods
We conducted a retrospective study between January 1st, 2013 to January 31st, 2021 in two departments at the National Hospital Centre of Pikine, Dakar: Internal Medicine/Endocrinology-Diabetology-Nutrition and Gynaecology-Obstetrics. The study included all women with DKD, known to be diabetic or not, with an ongoing pregnancy who were hospitalised during the study period. Medical records were the hospital digital archives. All records who had a diagnosis of diabetic ketosis in pregnancy were collected. the inclusion criteria were capillary blood glucose level > 2 g/l as determined using a blood glucose meter (Accu-Check®, Roche); ketonuria ≥2+ using a urine dipstick (Keto-Diastix®, Bayer). Acidosis was not mandatory for inclusion. Blood gas analysis is not available in our hospital. The diagnosis of acidosis was made on the basis of clinical criteria: kussmall’s dyspnea or impaired consciousness with no other apparent cause. Patients with incomplete records were excluded. For all participants included in the study we collected
· Epidemiological data: age, gravida, parity, type of diabetes, and risk factor for gestational diabetes mellitus.
· Diagnostic data: gestational age, symptom of ketoacidosis, capillary blood glucose, ketonuria, other obstetric complication, fetal viability at admission, precipitating factor, pregnancy monitoring, first trimester fasting glucose, glycated hemoglobin, serum electrolyte (Sodium, Potassium, Chloride) and renal function.
· Therapeutic data: ketoacidosis management, pregnancy management, and precipitating factor management.
· Monitoring data: duration of ketosis resolution and pregnancy outcome.
3. Results
Ten medical records of diabetes keto acidosis in pregnancy were collected and analysed.
3.1. Epidemiological Data
The mean age of the participants was 30.9 years (range 24 to 39 years). The mean gestational age at the time of ketoacidosis was 29.1 ± 2.77 weeks. Six patients were admitted in the third trimester of pregnancy and 4 in the second trimester. There were 6 multiparous, two nulliparous, and two primiparous patients. GDM was recorded in 8 patients and there were two cases of pre-GDM (one T1DM and one T2DM). At least one risk factor for GDM was found in patients with GDM: one patient previously had GDM and two women had first trimester blood sugar levels between 0.92 and 1.25 g/L. None of the participants had previously been screened for GDM. For the two diabetic patients, there was no regular follow-up and the pregnancies were not planned. No preconception measurement of glycated hemoglobin (HbA1c) level was available.
3.2. Diagnostic Data
Symptoms at admission were polyuria-polydipsic syndrome (n = 6), vomiting (n = 6), and abdominal pain (n = 2). Two pregnant women were completely asymptomatic on admission. Kussmaul dyspnea was present in 6 participants. One patient presented severe acidosis with impaired consciousness (Glasgow coma score 10/15). The average capillary glycaemia was 3.47 g/dL (range 2.62 to 4.95). Dipstick ketonuria was at 2+ in 4 patients, 3+ in two patients, and 4+ in 4 patients. None of the patients underwent arterial blood gas or lactate analysis. Infection was the most common precipating factor and was found in three patients. Diabetes was uncontrolled in two women with pre-GDM. Three patients had severe preeclampsia. Renal failure was present in one woman. Electrolyte disorders observed in the participants included hyponatremia (n = 2), hypokalaemia (n = 1), and hyperkalaemia (n = 1). Screening for vascular complications of diabetes was negative in all pregnant women. Intrauterine foetal demise (IUFD) was confirmed by obstetric ultrasound upon admission in three women.
3.3. Treatment Data
All patients received continuous intravenous (IV) insulin therapy, rehydration protocol and correction of electrolyte disorders. Specific management of the precipitating factor was conducted using antibiotics. Oxygenation was necessary for one patient. One patient with COVID-19 was transferred at the epidemic treatment centre for further care. For obstetric care, labour was induced in three women after lung maturation, an emergency caesarean section was performed in one woman with severe preeclampsia complicated by acute pulmonary oedema, and tocolysis was performed in two women due to a threat of premature delivery.
3.4. Monitoring Data
The mean time for the disappearance of ketones in urine was 24.1 hours. No cases of maternal death were noted. Pregnancy continued normally in 6 women, three of whom were subsequently lost to follow-up. The three remaining women all delivered by caesarean section, one at 32 weeks and the other two at over 37 weeks of gestation, with live and healthy neonates.
Foetal evolution during the episode of DKA was marked by one case of foetal distress complicated by intrauterine foetal demise. No cases macrosomia, or congenital malformations were noted. One patient gave birth to twins with low birth weight. Table 1 shows the epidemiological, diagnostic, and monitoring characteristics of the patients.
4. Discussion
Although DKA occurs at a higher frequency in women with T1DM, all pregnant women with diabetes are predisposed to DKA because pregnancy promotes insulin resistance, accelerated lipolysis, and a surplus of free fatty acids that can be shunted to ketone body production. Furthermore, high levels of circulating human chorionic gonadotropin can lead to nausea and vomiting and lead to higher risk of DKA early in pregnancy. In contrast, insulin resistance and metabolic demands increase significantly by the third trimester, which can precipitate DKA via hyperglycaemia and relative starvation. A major reason for earlier acidosis in pregnancy is lower acid buffering capacity; women who are pregnant
Table 1. Epidemiological, diagnostic, monitoring characteristics of study participants and pregnancy outcomes.
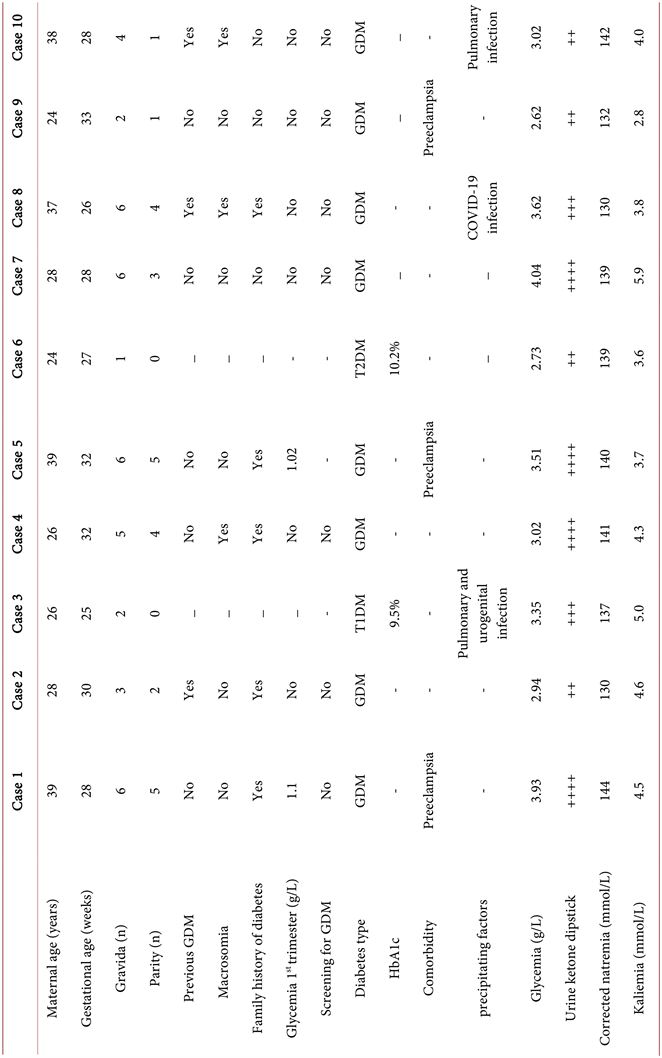
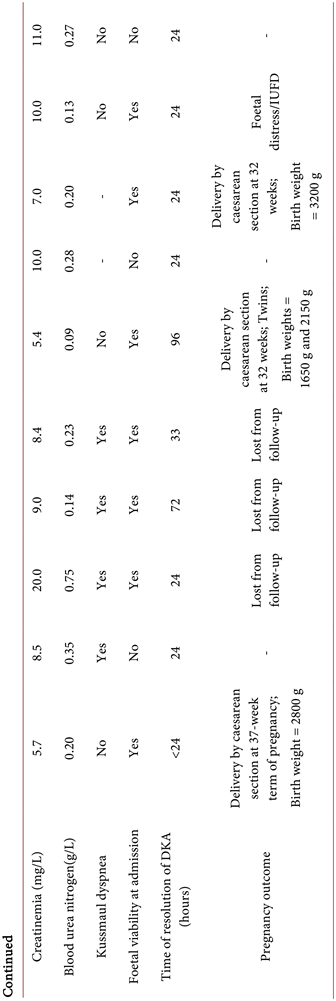
Laboratory standards for natremia = 135 - 145 mmol/L; laboratory standards for kaliemia = 3.5 - 5.3 mmol/L. DKA, diabetic ketoacidosis; GDM, gestational diabetes mellitus; IUFD, intrauterine foetal demise; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus.
have respiratory alkalosis with compensatory metabolic acidosis and, thus, lower bicarbonate levels [7] .
The largest published cohort of DKA in pregnancy is a case-control study from the United Kingdom that included 82 women [8] . The estimated incidence of DKA in pregnancy was 6.3 per 100,000 maternities. Most DKA episodes occurred in women with T1DM (70/82), but episodes in women with T2DM (5/82) and GDM (7/82) were also reported [8] . A similar distribution was reported in the US: out of a total of 58 gestational women with DKA collected over a period of 17 years, 48 had T1DM, three had T2DM, and there were 7 cases of GDM [9] . The occurrence of DKA in pregnancies complicated by GDM is rare, however, when it is encountered the possibility of unrecognised pre-existing diabetes should be strongly considered [10] .
Our results are different from the classically reported data which may be due to either a small study population and/or geographical location. Eight out of 10 study participants had GDM. Many of these patients could have had recognised pre-T2DM, especially considering that three of them already had presented an episode of GDM. Many women with GDM are pregestational diabetes patients that are first diagnosed in pregnancy, and women with true GDM are at risk for the development of diabetes mellitus (DM) later in life. Seventy percent of women with GDM will develop DM at some point in their lifetimes, and 40% to 50% of these women will develop DM within 10 years of GDM. Given the risk of adverse long-term health outcomes, postpartum glucose screening is recommended for women with a prior pregnancy complicated by GDM [11] [12] .
Seven study participants had at least one risk factor for GDM. None had previously been screened for GDM. According to the International Association of Diabetes and Pregnancy Study Groups criteria, the risk factors that correlated with the occurrence of GDM in the Dakar population were maternal age ≥ 30 years of age, multipartite women, previous GDM, and hormonal contraception [13] . In our cohort, 6 of 8 women were multiparous and three of 8 were previously diagnosed with GDM.
To decrease the risk of adverse pregnancy outcomes, women with DM considering pregnancy require tighter glucose control and medication review before attempting to conceive [14] [15] . The American Diabetes Association’s Standards of Care recommends that preconception counselling start at puberty and continue for all women of childbearing age [15] . Family planning discussions should occur and contraception should be prescribed until diabetes control is optimised for pregnancy. A HbA1c goal of 6.0% to 6.5% before conception is ideal unless maternal hypoglycaemia is of concern. In that case, the HbA1c target can be individualised to 7% or less if hypoglycaemia occurs at lower HbA1c levels [15] . Neither of the two patients with GDM had regular follow-ups and the pregnancies were not planned. Pregestational levels of HbA1c were unknown and therefore it was not optimally balanced on admission for DKA. The UK National Diabetes in Pregnancy Audit has shown that only one in 8 women with diabetes who become pregnant are optimised prior to pregnancy [8] . As found in our study group, the vast majority of cases with DKA in pregnancy emerge during the last trimester of gestation [1] [2] [3] [6] .
None of the patients underwent arterial gas analysis or lactate assay since these tests were not available in our hospital. This is a limitation of our study. Clinically, there was no symptom in the patients. The critical factors for DKA diagnosis were therapeutic insufficiency in patients with pre-GDM, unrecognised new-onset DM, and infection. Under these circumstances, and since DKA can occur at lower serum glucose levels during pregnancy (i.e., as low as less than 200 mg/dL; euglycemic DKA [1] ), pregnant women with DM are at risk of developing DKA more rapidly and at lower serum concentrations of glucose compared with non-pregnant women with DM. Maternal mortality secondary to DKA is also a risk but has significantly decreased in recent years to less than 1%. Foetal loss rates, however, are much higher and estimated to be between 10 to 35% [4] . During our study period, no cases of maternal death were noted, but three women had already lost their pregnancy on admission.
5. Conclusion
DKA in pregnant women is a rare situation in Dakar hospitals. It generally occurs in women in their thirties, with unknown risk factors for GDM or T2DM. It tends to occur in the second and third trimesters of pregnancy. Precipitating factors were dominated by infections. In patients with pre-GDM, DKA occurs in a context of uncontrolled diabetes with unplanned pregnancies. The obstetrical prognosis is poor with a high rate of severe preeclamsia and IUFD.