1. Introduction
Medicinal plants are used worldwide for the treatment of various diseases and disorders. Euphorbia L. (Euphorbiaceae) is the third largest genus of flowering plants, with rich pharmacological properties, and Euphorbia hirta L. is the species with the most useful records [1] . E. hirta is an annual medicinal herb with various medicinal properties, and it is distributed in many tropical continents (Asia, America and Africa) [2] . Indeed E. hirta is used widely in traditional Malay medicine to cure skin problems, amoebic dysentery, diarrhea, and ulcer [3] . In Nigeria, the exudates of the stem extracts of E. hirta have given satisfactory results in earache treatment [4] . In Burkina Faso, this plant is also used to treat digestive unrest, pregnancy-birth disorders, bacterial infections, diabetes, hypertension, visual disturbances, scorpion sting, parasitosis and allergies [2] [5] [6] . Pharmacological studies showed that extracts of E. hirta exerted antioxidant, antimicrobial, sedative anxiolytic, antiepileptic, anti-inflammatory, analgesic, antipyretic, antihistaminic, antiasthmatic, antidiabetic, anticancer, wound healing, gastrointestinal, diuretic, antiparasitic, immunological, hepatoprotective, galactogenic, angiotensin-converting enzyme inhibiting and anti-dipsogenic activities [7] [8] . Secondary metabolites such as flavonoids, steroids, terpenoids, coumarins, tannins, and polyphenols were isolated from E. hirta and characterized [9] [10] . Numerous active constituents from E. hirta with its pharmacological actions have been investigated by many researchers. However, few studies have listed these molecules and their properties [8] [9] [10] . The current review will summarize the most recent information on the compounds isolated from extracts of E. hirta, as well as its structures, pharmacological effects, mechanism of action and dosage efficiency.
2. Method
The present review covered the literature published prior to the year 2022. The information about Phytochemicals from E. hirta and its pharmacological properties was gathered from search engines like Google Scholar, NCBI, Scientific Research and Science Direct. Literature abstracts and full-text articles available from scientific revues were analyzed and bioactive compounds extracted from E. hirta were included in this review.
3. Taxonomy of Euphorbia hirta
Classification, according to Rhasid et al. [11] :
Kingdom: Plantae;
Phylum: Magnoliophyta;
Class: Angiospermae;
Order: Malpighiales;
Family: Euphorbiaceae;
Genus: Euphorbia;
Species: hirta.
4. Morphology
E. hirta is a slender-stemmed, annual hairy plant, spreading up to 40 cm in height, reddish or purplish in color, with many branches from the base to summit Photo 1. The leave with 1 - 2.5 cm long is opposite, elliptic-oblong to oblong-lanceolate, acute or subacute, dark green above, pale beneath. The fruits are yellow, three-celled, hairy, keeled capsules, 1 - 2 mm in diameter, containing three brown, four-sided, angular, wrinkled seeds [10] .
5. Distribution
Also called Euphorbia capitata Lam. or Euphorbia pilulifera Jacq. or Chamaesyce
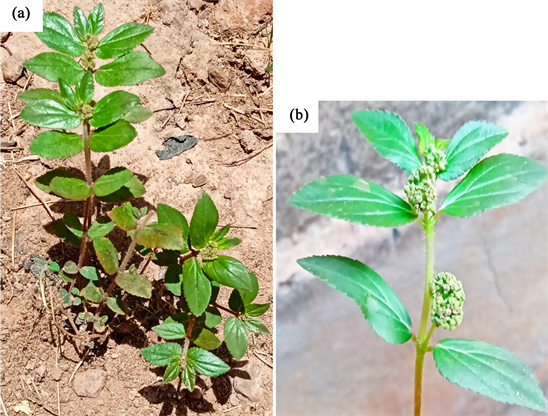
Photo 1. View of Euphorbia hirta L. (Kam, 2022): (a) Whole plant; (b) Fruits and leaves of plant.
hirta (L.) Millsp, E. hirta L. is distributed throughout America, Africa, Asia and Australasia. It is often found in waste places along the roadsides [7] [10] .
6. Bioactive Compounds Isolated from E. hirta
The fractionation, chromatographic separation and purification of different extracts of E. hirta have offered thirty-eight bioactive phytoconstituents. The sources, molecule class and solvents used for molecule extraction are organized in Table 1.
Phenolic constituents [12] , including flavonoids [10] , Phenolic acids [13] and tannins [14] , are the most represented class of bioactive molecules extracted. Furthermore, ten bioactive terpenoids from extracts were also obtained. Different parts of the plant were used to process the extractions. Aerial part and whole plant were frequently employed, and methanol was the most extraction solvent used, followed by ethanol.
7. Structures of Bioactive Phytochemicals Isolated
Most of the structures of bioactive phytochemicals in E. hirta were downloaded from PubChem database. The models of unknown structures were built on the ACDLabs202020_ChemSketch software. The structures of 38 drug molecules are illustrated in Figure 1.
8. Biological Potential of Phytochemical
The bioactive compounds isolated from E. hirta, have demonstrated fourteen biological activities recorded. Table 2 presents mechanisms of action of compounds
![]()
Table 1. Phytochemicals isolated from E. hirta.
![]()
![]()
![]()
Figure 1. Structure of 38 bioactive components suggested from PubChem
![]()
Table 2. Mechanisms of compounds isolated from E. hirta.
isolated from E. hirta. Quercetrin following by quercetin and β-amyrine were the most characterized biomolecules. They had properties such as anti-diabetes, anti-inflammatory, antimalarial, anticancer, anti-snake venom, antimicrobial, antidiarrhoeic and antistress. Otherwise, not identified lignans from ethanol extract of E. hirta has demonstrated an anticancer activity against cell lines Hep G2 with IC50 value of 7.2 ± 0.17 and 8.5 ± 0.36 µM [40] . The peptide fractions from protein hydrolysate of E. hirta have Shown also a cytotoxicity against a gastric carcinoma cell line (KATO-III, ATCC No. HTB103) at 100 μg peptides ml−1 [41] .
8.1. Anti-Diabetes
The following new prenylated flavonoids, Quercetrin, 3’,4’-Dimethoxyquercetin, Hirtacoumaroflavonoside and Hirtaflavonoside-B, isolated from methanolic extract of E. hirta were studied for their antidiabetic activity by Sheliya et al. [27] . They inhibited in vitro α-glucosidase with IC50 values of 0.151, 0.182, 0.022 and 0.071 mM, respectively. They also regulated the postprandial hyperglycemia in rats at 10 mg/kg. The work of Le et al. [25] on the effect of Quercetrin from ethanol extract of E. hirta, on endoplasmic reticulum stress-induced cell death in mouse pancreatic β-cell lines, revelated its strong cell-protective effect with the cell viability of 78 % at the dose of 10 µg/mL. Other flavonoids (Quercetin, rutin, myricitrin, cyanidin 3,5-O-diglucoside, Pelargonidin 3,5-diglucoside) and the terpenoids such as α-amyrine, β-amyrine, taraxerol have demonstrated a high binding affinity (<−8.0 kcal/mol) to protein relating diabetes Type 2 in silico [42] .
8.2. Anti-Viral
Myricitrin is a flavonoid from 50% ethanol/methanol extract of E. hirta. It inhibited Japanese encephalitis virus at 100 μM [28] . Galloylquinic acid (phenolic acid) was effective against nonstructural proteins (NS1, NS3) and envelope protein domain III of ZIKA virus in silico [43] . Euphorbianin and rutin are other flavonoids investigated in silico. They showed high binding affinity against protease Mpro, RNA-dependent RNA polymerase RdRp of SARS-CoV-2 [44] .
8.3. Anti-Inflammatory
The study of Shih et al. [35] showed that the anti-inflammatory effect of ethanolic extract of E. hirta, is mediated through its terpenoid component, β-amyrin. It blocked the iNOS protein functions (at 0.025 mg/ml) and the nitric oxide (NO) production (at 0.0125 mg/ml) on the LPS-induced RAW 264.7 cells. β-amyrin and other terpenoids (24-methyl encycloartenol and β-sitosterol) isolated from n-hexane extract of E. hirta aerial parts, exerted significant inhibition effects on TPA-induced inflammation in ear to the mice with ED50 value of 0.12, 0.26 and 0.14 mg/ear respectively [36] . The methanol extract of E. hirta aerial parts, rich with two flavonoids (Quercetrin, Rhamnetin) and two phenolic acids (Ferulic acid, Gallic acid), was standardized and designated as PM 251 by Subbiah [18] . PM 251 has proved to be a significant COX-2 (Cyclooxygenase) enzyme inhibitor in vitro. It was also revealed to be a good anti-inflammatory agent in two acute (turpentine-induced arthritis and formalin-induced experimental peritonitis) and the sub-acute (cotton pellet-induced granuloma) models of inflammation in the doses of 100 mg/kbw, 200 mg/kbw and 400 mg/kbw body weights when given orally to rats.
8.4. Anticancer
Afzelin, Quercetrin and Myricitrin are flavonol glycosides isolated from methanolic extract of E. hirta aerial parts. They exhibited a cytotoxic property against human epidermoid carcinoma KB 3-1 cells with IC50 values of 276.1, 88.2 and 156.4 µg/ml, respectively [24] . A mixture of two terpenoids, 25-hydroperoxycycloart-23-en-3β-ol and 24-hydroperoxycycloart-25-en-3β-ol, from CH2Cl2 extract of E. hirta leaves have demonstrated cytotoxicity activities against a human cancer cell line, colon carcinoma (HCT 116) at 4.8 μg/ml; and against non-small cell lung adenocarcinoma (A549) at 4.5 μg/ml [34] . According to Paulpandi et al. [45] , Quercetin is a flavonoid isolated from E. hirta leaves, which showed reduction in human breast adenocarcinoma MCF-7 cells viability with IC50 value of 2 μM.
8.5. Antimalaria
The flavonol glycosides, Afzelin, Quercetrin, and Myricitrin from methanolic extract of E. hirta aerial parts, have been reported to reduce the proliferation of Plasmodium falciparum with IC50 values of 1.1, 4.1 and 5.4 µg/ml respectively [24] . The findings of Shah et al. [46] also indicated that flavonoids such as Isorhamnetin and Pinocembrin had significant inhibitory activity against plasmepsin protease in silico approach.
8.6. Antimicrobial
According to the work of Ragasa and Cornelio [34] , three triterpenes isolated from CH2Cl2 extracts of E. hirta were found to exhibit antimicrobial activities. Indeed, Taraxerol from stems extract was active against Pseudomonas aeruginosa and Staphylococcus aureus at 30 mg. In addition, the mixture of 25-hydroperoxycycloart-23-en-3β-ol and 24-hydroperoxycycloart-25-en-3β-ol from leaves extract was active against the bacteria: P. aeruginosa, S. aureus, Escherichia coli and fungi: Candida albicans and Trichophyton mentagrophytes at 30 mg. Moreover, two flavonoids, Quercetin and Kaempferol, identified in the bound flavonoids from 80% hot methanol extract of stems extract, showed an activity against the bacteria: E. coli, P. aeruginosa, Proteus mirabilis, S. aureus and the fungi: Aspergillus flavus, Aspergillus niger, T. mentagrophytes and C. albicans at 1 mg/disc [22] . The evaluation of antibacterial and antifungal activities of two triterpenoids, Taraxerone and 11α, 12α-oxidotaraxerol isolated from petroleum ether extract of E. hirta, revealed their efficiency against Bacillus subtilis, B. cereus, B. megaterium, Sarcina lutea, S. aureus, E. coli, Shigella dysenteriae, S. sonnei, S. shiga, S. boydii, S. flexneriae, P. aeruginosa, Salmonella typhi, Klebsiella sp. Aspergillus flavus, A. niger, Penecillum sp. Trichoderma viride, C. albicans, Botryodiplodia theobromae with MIC value from 64 to 128 μg/ml [14] . The methanol extract of E. hirta aerial parts, have offered two antibacterial compounds, Caffeic acid (Phenolic acid) and Epicatechin 3-gallate (Flavonoid), which targeted both cell wall and cytoplasmic membrane of P. aeruginosa with MIC value at 15.6 and 31.3 µg/mL, respectively [12] [19] . The screening by TLC of ethyl acetate extract of E. hirta leaves, which had an effectiveness against MRSA: S.aureus B39 (MIC = 25mg/ml), showed that the anti-MRSA spots were identified as Hydroquinone (Diphenol) and O-coumaric acid (Phenolic acids) [15] . A hydrolyzable tannin, Euphorbin C, derived from E. hirta, was effective against Helicobacter pylori with MIC value from 25 to 50 µg/ml [33] .
8.7. Anti-Atherosclerosis
β-Amyrin, an active terpenoid of E. hirta, purchased from the extrasynthese (Taipei), inhibited the atherosclerotic initiation induced by pro-inflammatory cytokines in SVEC4-10 endothelial cells at 0.6 and 0.3 µM [47] .
8.8. Antidiarrhoeic
Quercetin is an antidiarrhoeic flavonoid constituent from acetone:water (7:3) extract of E. hirta. It decreased both the total number of faeces and the number of diarrhoeic faeces induced in mice by castor oil at content ranging from 12.5 to 100 mg/kg [13] .
8.9. Anti-Stress
The funding of Tiwari et al. [23] has revealed the anti-stress potential of Quercetin, a flavonoids isolated from hydroalcoholic extract of E. hirta leaves. Mice pretreated with this molecule at the dose of 25, 50 and 100 mg/kg showed significant improvement in the swimming time, a rise in the time spent in open arm and a decrease in the time spent in the closed arm, compared to the control group.
8.10. Anti-Asthmatic
Taraxerol is a triterpene isolated from ethanolic extract of E. Hirta stems. It possesses an anti-asthmatic property by the inhibition of the contractile effect of histamine in guinea pigs at 100 and 200 mg/kg [37] .
8.11. Anti-Thrombotic Disorders
The terpenoids, Hirtin isolated from latex of E. hirta, exhibited in vitro Azocaseinolytic and thrombin-like activities at 5 µg, gefibrinogenolytic and fibrinolytic properties at 2 µg [38] .
8.12. Antioxidant
Hydroxyl cinnamic acid derivatives are phenolic acids and antioxidants isolated from aqueous extract of E. hirta leaves. They exhibited an effective value of EC50 150 g/ml in the protective interaction with reference bovine serum albumin protein (BSA) against metal-catalyzed oxidation (MCO) system [20] . The following molecules Methyl-3-(3,5-ditertbutyl-4-hydroxyphenyl) propionate, a hydroxyphenylcarboxylic acid esters from methanol extract of leaves; and twenty new chebulic acid and brevifolincarboxylic acid derivatives (phenolic acids) from ethanol extract of plant aerial parts, acted as an antioxidant by DPPH radical scavenging activities in vitro with IC50 = 30.02 ppm and EC50 values from 2.2 to 15.8 μM respectively [21] [39] .
8.13. Anti-Snake Venom
The bioassay-guided fractionation of methanol extract of E. hita yielded Pyrogallol (triphenol) and Quercetrin (flavonoid), with protective effect against snake venom [16] [26] . Pyrogallol inhibited in vitro Naja naja venom protease activity at 1:40 w/w (venom:Pyrogallol). Concerning the quercetrin (QR), in vitro experiments indicated that protease, phospholipase-A2 and hemolytic activities of Naja naja venom were inhibited completely at a ratio of 1:20 w/w (venom:QR). A significant inhibition of hyaluronidase activity was also observed at 1:50 w/w (venom:QR).
In addition, in vivo study revealed that Quercetrin exhibited at 1:20 w/w, the inhibition of hemorrhage and edema induced in mice. It extended the survival time of mice injected with snake venom.
8.14. Anti-Hemorrhoid
Rutin, a flavonoid isolated from ethanol extract of E. hirta, showed remarkable healing on croton oil-inducing hemorrhoids in Wistar Albino rats at 100 mg/kg [29] .
9. Conclusion
In this review, we have summarized the structures and properties of 38 bioactive phytochemicals isolated from E. hirta. Our studies showed that this plant could be a promising source of novel drug candidates. Further investigations are necessary to understand the relationship existing between phytochemicals isolated and their activities.
Authors’ Contributions
Conceptualization: R.N.M. and S.E.K.; reviewed the literature and writing: all authors; validation investigation: R.N.M. All authors have read and approved the final manuscript.
Funding
This study received no external funding.