1. Introduction
The decrease in water level in the Aral Sea, caused increased salinization, and caused a number of consequences such as land degradation, desertification, which had a negative impact on biodiversity in the coastal region. The salinization of the adjacent area is one of the environmental problems studied not only by domestic scientists [1] , but also worldwide [2] [3] [4] . According to the resolution adopted at the UN General Assembly, the Aral Sea area is a zone of ecological innovation and technology (https://lex.uz/docs/5538603), constituting one of the strategic objectives of the national scale in the Republic of Uzbekistan.
The area of exposed seabed formed the youngest desert Aralkum. Currently, the area of the dried seabed is 4.7 million ha (2017) [5] , with more than 6 million ha (2021) belonging to the sandy-saline type of arid desert, and the area is increasing [6] . A significant part of these saline lands falls on the territory of Uzbekistan, here almost 3 million hectares are subjected to salinization, 53% of the dried seabed [1] .
The use of plant resources in obtaining medicines and locally useful species is an important step in the creation of a sustainable raw material base. The promising use of species of the Chenopodiaceae family, as a medicinal and food raw material, makes it relevant to investigate new promising sources of polyphenols to identify the chemical structure of compounds and their biological activity. Therefore, in order to obtain more stable and competitive salt-tolerant plants, it is necessary to take into account the structural, physiological and biochemical characteristics of halophytes. Thus, a positive correlation has been found between an increase in the concentration of salts in the medium and an increase in secondary metabolite production [7] .
All medical products applied in modern medicine for treating viral diseases are medicines of synthetic nature, and have some side effects. This shows the urgency and relevance of creating drugs based on natural compounds. To solve this problem, it is necessary to search for new promising sources of polyphenols, isolation and determination of their chemical structures, revealing their biological activity.
According to the above, the material for our research was species belonging to the family Chenopodiaceae which has not been systematically studied in Uzbekistan. In this regard, the study of chemical composition, development of methods of isolation of potentially biologically active substances, research of biological activity and development of new drugs and phytopreparations are relevant. Salt-tolerant plants have huge industrial reserves on the territory of the Republic of Uzbekistan.
Plants of Chenopodiaceae family are succulent, have mostly salt accumulating effect, live on saline and alkaline soils, occupy vast areas of deserts and semi-deserts of Uzbekistan and also play an important role in plant landscapes and are the sources of biologically active substances. Therefore, the study of chemical composition and development of methods of biologically active substance extraction with subsequent biosurfactant screening are of great interest today and are particularly relevant, since they will contribute to the creation of highly effective domestic phytopreparations with a wide range of pharmacological action.
Globally, plants of the family (Chenopodiaceae) include about 100 genera and 1400 species, of which 24 genera and 59 species are found in the Southern Aral Sea [8] .
Thus, the study of the chemical composition of some plants growing in saline areas, isolation and determination of the structure of biologically active substances and development of phytopreparations based on them are undoubtedly relevant.
Thus, the chemical composition of representatives of the Chenopodiaceae family as a source of biologically active substances is of great interest both in the world and Uzbekistan, such plants include Halocnemum strobilaceum (Pall.) M. Bieb. of the monotypic genus. A number of secondary metabolites such as terpenoids, steroids, flavonoids, and tannins have been isolated from this species and are used as a medicinal plant in the treatment of skin diseases, helminthiasis and warts. The plant also has cytotoxic properties and is widely used as an antibacterial and antiviral agent. It is represented by various amino acids, carbohydrates, phenolic compounds (flavonoids, isoflavones, xanthones, tannins, etc.), essential oils, etc. [9] . In this regard, the aim of our work is to establish the composition of polyphenols and antioxidant activity of Halocnemum strobilaceum plants.
2. Materials and Methods
The object of the study was a salt-tolerant succulent hyperhalophyte of Chenopodiaceae family. The above-ground part of plant H. strobilaceum was collected in natural conditions of salt marshes in Karakalpakstan (Muynak district, N 43.693355 E 59.039325), during field visits in July 2021 (Figure 1).
A shrub often forms a cushion densely pressed to the ground, with opposite branches, succulent annual shoots and undeveloped, glabrous-like shoots, and leaves [9] .
In the growing area of the H. strobilaceum objects we studied, the soil had the following salt composition: Ca(HCO3)2—0.024%; CaSO4—1.064%; MgSO4—0.158%; Na2SO4—0.413%; NaCl—0.911%. The soil is dominated by gypsum—41.5% (of the sum of salts), sodium chloride—35.4%, glauber salt—16.1%, a lot of “bitter salt” (MgSO4)—6.15% toxic to plants [8] .
Thus, at the collection sites the chloride-sulphate type of salinization with insignificant alkalinity, constant presence of gypsum (CaSO4) and glauber salt (Na2SO4) in sufficiently large quantities in the soil profile is noted. Important feature of
![]()
Figure 1. Halocnemum strobilaceum in natural habitat. (a) General view; (b) Generative shoot in flowering phase.
the salt composition of the soil is abundance of Na2SO4.
Egyptian scientists [10] [11] [12] identified flavonoids isolated from chloroform and ethyl acetate fractions of water-alcoholic extract of Halocnemum strobilaceum as chrysoeryol, 7-O-galactoside luteolin, 7-O-rhamnoside quercetin, luteolin and coumarin, namely scopoletin. Their identity was confirmed by t.p., TLC, PC, UV, 1-H NMR and MS analysis. The ethyl acetate extract has strong antioxidant activity, as do the isolated flavonoids, compared to trolox (the standard antioxidant compound).
Metabolomic analysis of Halocnemum strobilaceum bioactive ethyl acetate fraction against UPLC/PDA/ESI-MSn-based cancer cell lines; PC3, MCF-7 and A549 showed that the species is rich in flavonoid glycosides with fragments of quercetin, isoramnetin and icaritin. These results suggest that the ethyl acetate fraction derived from Halocnemum strobilaceum may act as a rich source of natural antioxidants with potent anticancer activity.
In tunnis [13] identified twelve phenolic compounds in H. strobilaceum, with rosmarinic acid and 3,4-dimethoxybenzoic acid successfully identified. The main acids identified by GC/MS were fatty acids. The differences in antioxidant and antimicrobial activity between the selected fractions were mainly due to the different biologically active molecules contained in each fraction. Such characteristics will be of paramount importance to enhance the value of this halophyte and its use in the cosmetic and pharmaceutical industry as a valuable source of antioxidant molecules.
Miftakhova et al. [14] isolated flavonoids and flavonoid glycosides in Halocnemum strobilaceum, 5 substances of which were identified (5) 3,4’,5-trihydroxy-3’-metoxyflavone 7-O-a-D-glucosaminopyranoside.
Akzhigitova [9] showed that Halostachys belangeriana (Moq.) Botsch. plants contain active alkaloid halostachine (ephedrine analogue); water extract from fresh Halocnemum strobilaceum (Pall.) M. Bieb., has insecticidal properties; Kalidium caspicum (L.) Ung.-Sternb., contains a high percentage of ash and potash. In the flora of Uzbekistan, there are scarce data on the determination of polyphenols of desert plants.
These results indicate a suitable solvent for biophenols extraction with this halophyte, which is an important source of remarkable potential secondary metabolites with original and interesting antioxidant and anticancer abilities.
In order to isolate polyphenolic compounds from plant material in high yield, factors affecting the extraction process were studied. It has been stated that the optimum condition of extraction of polyphenol compounds sum is 3-fold extraction in 70% acetone in the ratio of raw material extractant 1:6 at temperature 45˚C for 2 hours.
Purification of plant raw materials from lipophilic compounds was carried out 3-fold extraction with chloroform at 45˚C. After the raw material was dried under a desiccator at room temperature until the smell of solvent disappeared, 3-fold extraction was carried out with 70% aqueous acetone.
The obtained extracts were thickened at a rotary evaporator to an aqueous residue. In a separating funnel, the residue was treated several times with ethyl acetate (1:6 ratio) and an ethyl acetate extract was obtained.
By thickening the ethyl acetate fraction with a rotary evaporator and ethyl acetate concentrate was obtained. The ethyl acetate concentrate was dried with anhydrous sodium sulfate (Na2SO4) and left for 24 hours. After filtration the concentrate was precipitated with chloroform in a 1:4 ratio. The precipitate was dried in a vacuum desiccator at 40˚C.
To study the component composition of substances were analyzed by HPLC-mass spectrometry. Separation was performed by HPLC (Agilent Technologies-1260, USA) on a 2.1 × 150 mm (3.5 µ) Eclipse XDB column (Agilent Technologies, USA).
We used a mixture of eluents A-0.05% formic acid buffer solution with water, B-acetonitrile and C-isopropanol with a gradient of 0-min 18% B + 2% C, 15-min 72% B + 8% C, 18-min 18% B + 2% C. The flow rate is −0.25 ml/min.
Mass spectra of substances were obtained by ESI mass spectrometry (electrospray) using a 6420 Triple Quad LC/MS mass spectrometer (Agilent Technologies, USA).
Registration of mass spectra of samples was carried out with negative ionisation. Parameters of the mass-spectrometer were chosen: scanning range 100 - 2200 m/z, dryer gas flow rate 3l/min, gas temperature 300˚C, gas pressure at the atomizer needle 20 psi, evaporator temperature 300˚C, capillary voltage 4000 V.
3. Results and Discussion
3.1. Polyphenol Analysis by HPLC
Polyphenols were analyzed on an Exlipse XDB C18 5 µm (4.6 × 150 mm) sorption
![]()
Figure 2. The content of the highly active substances was analysed by HPLC
column equipped with an Agilent Technologies 1200 series DAD detector (Figure 2). Flow rate 1 ml/min, mobile phase: A—0.1% phosphate buffer, V—acetonitrile. Gradient % V/min: 5%/0 - 5 min, 60%/30 - 35 min, 5%/45 min. Polyphenols were detected at 269 nm. The temperature in the column was 250˚C.
HPLC analysis of polyphenols: Exlipse XDB C18 5 µm column (4.6×150 mm). Flow rate 1 ml/min, mobile phase: A—0.1% phosphate buffer, V—acetonitrile. Gradient %V/minute: 5%/0 - 5 min, 60%/30 - 35 min, 5%/45 min. UV 269 nm (Table 1).
3.2. Antioxidant Activity
To evaluate the antiradical activity (ARA) in this work, a spectrophotometric
![]()
Table 1. Identification of polyphenols of the hyperhalophyte H. strobilaceum.
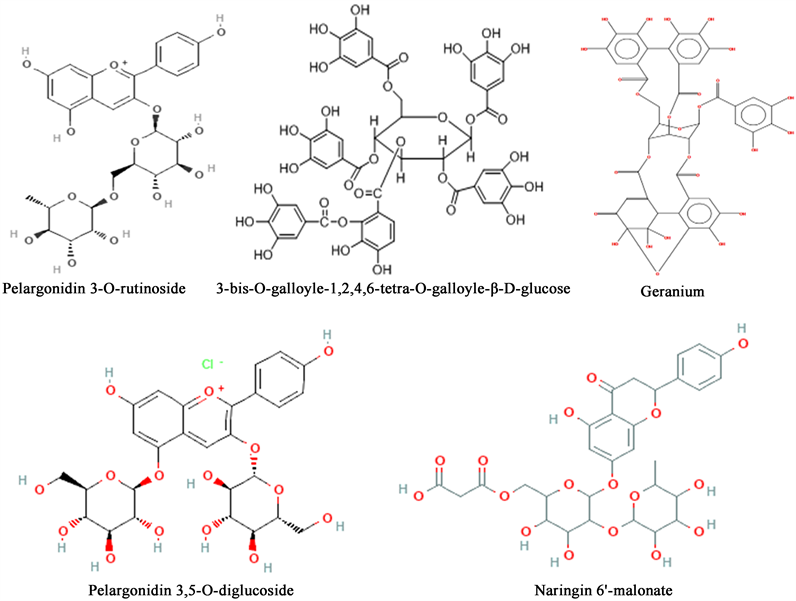
technique was used to measure the kinetics of stable radical reduction of 2,2-diphenyl-1-picrylhydrazyl (DPPH) molecules by antioxidants. The compounds studied were dissolved in water at a concentration of 1 mg/ml.
As antioxidants may have different mechanisms of action, it is advisable to study their activity using different methods. In this work, the ARA of the extracts was evaluated against the free radical DPPH. When the studied compounds were added to an alcohol solution of DPPH, the free-radical molecules were converted into a non-radical form, and the intensely purple DPPH solution became discoloured.
To compare the ARA of the test samples, a concentration of 50 µl of each extract was chosen from a prepared solution of 1 mg of the substance in 1 ml of alcohol.
From the experimental data, it follows that extract has the highest free radical quenching ability. Stable radical (DPPH) as well as parameter t50, the time required by the studied preparations to decrease the initial radical concentration by 50%, were used to quantitatively estimate the antiradical activity. In the reaction of DPPH with extracts, t50 at 20˚C is 60 ± 4.3 for a sample of this plant.
4. Conclusions
In this connection, considering that the extract from Halocnemum strobilaceum has high antiradical activity, it seems important to study the possibility of correction of disturbances in oncological, diabetic and other non-infectious patients and consider them as promising drugs.
The obtained analyses show that this desert plant will allow creating in the future of a new range of promising biologically active substances, as well as using effectively the plant cover around the Aral.
Acknowledgements
This work was carried out within the project AL-632204135.