Anti-Biofilm, Anti-Quorum Sensing, and Anti-Proliferative Activities of Methanolic and Aqueous Roots Extracts of Carica papaya L. and Cocos nucifera L. ()
1. Introduction
The upsurge of resistant microbial pathogenic strains and cancer has become a global public health concern [1] [2] . Indeed, antimicrobial resistance is recognized as one of the top ten growing public health threats, currently estimated to account for more than 700,000 deaths annually. If proper care is not taken, this figure could rise substantially to 10 million by 2050, thus leading our planet to the post-antibiotic era [3] . Likewise, the development of resistance to chemotherapy continues to be the main barrier in the treatment of cancer patients [4] . Consequently, there is a significant need for newer agents with low susceptibility to common drug resistance mechanisms to improve response rates and potentially extend survival [4] . Over the years, nature has been a source of medicinal agents. The extraction, isolation, and identification of plants containing phytochemicals have led to the discovery of new therapeutics in the research and development sector of the pharmaceutical industry [3] . Therefore, since ancient times a plethora of plant extracts and active biological compounds have been widely identified and documented for the treatment of infectious and cancerous diseases [5] [6] [7] [8] . Thus, curcumin from Curcuma Longa L. (Zingiberaceae) has been used in the treatment of infectious diseases. Taxol a very potent chemotherapeutic compound derived from the bark of Taxus brevifolia (Taxaceae) is very active in the treatment of cancer. Morphine from Papaver somniferum L. (Papaveraceae) is a powerful analgesic [3] [9] . Furthermore, plants offer a unique and renewable resource for the discovery of new therapeutically active biomolecules thanks to the structural and biological properties of their constituents [10] . In the World Health Organization “Traditional Medicine Strategy” for the period 2014-2023, it is recognized that traditional medicines are often underestimated in terms of the role they can play in health care systems. She further states that medicinal plants may in some countries be the main source of health care or even the only health service available, especially for rural populations [11] . The main advantages of using plant-derived medicine are that they are safer than synthetic alternatives, providing therapeutic benefits and affordable treatment [5] [12] .
This study is a continuation of our previous work on the energy capacity, antioxidant and anti-inflammatory properties of C. papaya and C. nucifera root extracts [13] . Therefore, in the present report, we set out to study the effect of plant extracts on biofilm formation on a few bacterial strains, namely Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Streptococcus mutans, as well as their ability to interfere with the bacterial quorum sensing system. The cytotoxicity of the extracts on prostate cancer cell lines and the total content of triterpenes and sterols were also provided.
2. Material and Methods
2.1. Study Area
This study was carried out between from October, 2019 to November, 2020 at the Department of traditional medicine of Research Institute of Health Sciences, the Laboratory of Applied Biochemistry and Chemistry of the University Joseph KI-ZERBO; and the Pietro Annigoni Biomolecular Research Center (Ouagadougou, Burkina Faso).
2.2. Standards and Reagents
All chemicals and reagents used to carry out the experiments were of analytical grade.
N-hexanoyl-L-homoserine lactone (HHL), Crystal violet, iodonitrotetrazolium chloride (INT), salicylic acid, agar agar, Brain Heart Infusion (BHI) broth and Luria-Bertani (LB) broth, blue trypan, 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) and Phosphate buffered saline PBS were purchased from Sigma® (St Louis, USA). Dimethyl sulfoxide (DMSO), methanol, glycerol, hydrochloric acid, isopropanol, acetic acid, and ethanol were procured from Prolabo (France). Culture medium RPMI-1640, Penicillin, and streptomycin were purchased from Invitrogen (Oslo, Norway); and Fetal calf serum from Biowest, Nuaillé, France.
2.3. Plant Material
The Fresh roots of Carica papaya were collected in September of 2018 from the area of Dedougou/Burkina Faso. The plant was taxonomically authenticated and a voucher specimen bearing the number T4316 was deposited at the Plant Biology and Ecology Laboratory of the University Joseph KI-ZERBO, Burkina Faso. Fresh roots of Cocos nucifera were provided by a tradipractician and were also authenticated.
2.4. Preparation of the Plant’s Extracts
The roots of two plants were shade-dried at room temperature. Roots powder was obtained by a blade crusher. Two extracts (aqueous and methanolic) of each plant were prepared from the powdered roots.
The aqueous maceration was obtained by dispersing 50 g of each powder in 500 mL of distilled water. After 24 hours at room temperature, the extract was filtered, freeze at −54˚C and freeze-dried under pressure reduced to 0.151 mbar at −49˚C during 72 hours.
To prepare the methanolic maceration, the same method was used. Thereafter, the extract was filtered, concentrated using a rotary vacuum evaporator, and put in an oven until completely dry.
2.5. Dosage of Triterpenes and Sterols
The total content of triterpenes and sterols was determined colorimetrically using the procedure described by Chang et al. [14] with slight modifications. 30 μL of vanillin—glacial acetic acid 5% solution was mixed with 20 μL of total genins previously extracted (5 mg/mL) and dissolved in methanol. 100 μL of perchloric acid was then added. The mixture was placed in a water bath at 60˚C for 45 min, then cooled in an ice water bath for a few minutes. After the addition of 450 µL of glacial acetic acid, the absorbance of each sample solution was measured using a spectrophotometer (BioTeck instruments, USA) at 548 nm against a curve of ursolic acid (y = 0.1259x + 0.0653; R2 = 0.99) for the triterpenes (Figure 1). The absorbance was also measured at 640 nm against a cholesterol calibration curve (y = 0.0744x − 0.0056; R2 = 0.99) for sterols (Figure 2).
The analyzes were carried out in triplicate and the results were evaluated respectively in mg Ursolic Acid Equivalent per g of extract (mg UAE/g extract) and mg Cholesterol Equivalent per g of extract (mg CE/g extract).
![]()
Figure 1. Calibration curve of Ursolic acid.
![]()
Figure 2. Calibration curve of Cholesterol.
2.6. Antimicrobial Testing
2.6.1. Bacterial Strains Used in Assays and Growth Conditions
The pathogens used for the experiment were: Escherichia coli ATCC (American Types Collection Culture) 25922, Pseudomonas aeruginosa PAO1, Staphylococcus aureus ATCC 43300, Streptococcus mutans ATCC 25175, and Chromobacterium violaceum CV026. The strains Escherichia coli, Pseudomonas aeruginosa, and Chromobacterium violaceum were grown in LB broth medium while Staphylococcus aureus and Streptococcus mutans were grown in BHI liquid medium. All strains were grown at 37˚C except CV026 grown at 30˚C.
2.6.2. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
The MIC values of the extracts were determined using the 96-well plate method according to Eloff [15] . To do this, a range of dilutions of the extracts was carried out on a sterile microplate from a stock of 2 mg/mL final concentration. To 100 µL of each dilution was added 100 µL of inoculum (106 CFU/mL) prepared in the corresponding culture. The microplates were then incubated for 24 hours at 37˚C after which 30 μL of p-iodonitrotetrazolium (INT) (2 mg/mL) was added to each well. 30 min after the addition of INT in the dark, the MIC of the extracts was visibly deduced. Living microorganisms reduce the INT by producing a pink color [16] .
The MBC was determined using samples from the MIC microplate. A loopful of inoculum was collected from the well with the concentration before the well that had been read as the MIC. They were then streaked onto agar medium. The Petri dishes were incubated for 24 hours at 37˚C. The CMB was visibly deduced as being the first dish devoid of bacteria [17] .
2.6.3. Biofilm Formation Using Crystal Violet Assay
Anti-biofilm activity of C. papaya and C. nucifera extracts on the strains (Escherichia coli, Streptococcus mutans, Pseudomonas aeruginosa, Staphylococcus aureus) was carried out according to crystal violet method on 96-well plates, based on Vandeputte et al [18] . An appropriate dilution (100 μL) of each strain overnight culture was added to the corresponding culture medium supplemented (80 µL) with 20 μL of extract, salicylic acid (reference substance), or DMSO (100 μg/mL, final concentration). After incubation of the plates, the absorbances were read at 600 nm to follow the bacterial growth compared to the negative control (DMSO). The supernatant from each well was then removed and the biofilms were gently washed with distilled water, fixed with methanol for 15 min, and dried. 0.1% crystal violet (in water) was added to each well and the plates were incubated for 30 min. After removing the crystal violet, the wells were rinsed with distilled water and 200 μL of acetic acid (33% in water) was added to dissolve the crystal violet. The absorbances of the solution were read at 590 nm and the biofilm/bacterial growth ratio (590 nm/600 nm) was determined. The percentage inhibition of the extracts was calculated by the following formula:
% Inhibition = [(Ac − At)/Ac] × 100
Ac: absorbance of negative control; At: absorbance of the tests (extracts, reference).
2.6.4. Violacein Production in Chromobacterium Violaceum Assay
C. violaceum CV026 was used to determine anti-quorum sensing (QS) activity by quantifying QS-controlled violacein production. Thus, the extracts were evaluated by their ability to inhibit the production of violacein in C. violaceum according to the method described by Choo et al. [19] adapted by Ouedraogo et al [20] . Briefly, C. violaceum was grown for 24 h at 30˚C. CV026 was diluted and introduced into a 12-well plate, then the extracts and reference (salicylic acid) were added to have a final concentration of 100 μg/mL in the presence of HHL. The plates were incubated for 24 hours at 30˚C. Bacterial turbidity was measured at 600 nm to assess bacterial growth, then 1 mL from each well was taken and introduced into tubes to quantify the violacein. The solution was vigorously vortexed to solubilize the violacein then the supernatant was removed and 1 mL of DMSO was added to the pellet. After centrifugation, 200 μL of the supernatant containing the violacein are introduced into a 96-well microplate. The production of violacein was quantified by measuring the absorbance at 585 nm and the percent inhibition was calculated against the negative control (DMSO). The ratio between 585 nm and 600 nm was also determined.
2.7. Antiproliferative Activity
2.7.1. Cancer Cell Lines and Culture Conditions
The cell lines used were adherent LNCaP (androgen-sensitive) and PC-3 (androgen-resistant) prostate cancer cells supplied to CERBA/LABIOGENE laboratories by the GReD Laboratory (University Clermont-Auvergne, France). The LNCaP (Lymph Node Cancer of the Prostate) and PC-3 cell lines were cultured and maintained at 37˚C in a humidified incubator with 5% CO2 in 75 cm2 cell culture flasks, in RPMI-1640 (Roswell Park Memorial Institute) medium supplemented with 10% Fetal Calf Serum, 1% Penicillin-Streptomycin and 1% L-Glutamine.
2.7.2. Cytotoxicity Assay
The MTT assay was used to measure cell survival. Briefly, 10,000 cells/mL were seeded for 24 h in a 96-wells microplate. After 24 h, the methanolic extracts of Carica papaya and Cocos nucifera were dissolved in complete culture medium RPMI-1640 (1% DMSO) and a dilution range was achieved of concentrations ranging from 500 µg/mL to 1.95 µg/mL. 50 µL of each extract was then brought into contact with the cells in the wells and the plates were incubated again. After 72 h incubation, the number of living cells was measured as described by Bayala et al. [21] using a microplate reader type Bio-Rad 11885 at 490 nm. Data are the results of three independent experiments for each cell line. Growth inhibition was calculated as follows:
% Inhibition = 100 − [(Extract absorbance − blank absorbance)/(Control absorbance − blank absorbance)] ×100.
2.8. Statistical Analysis
The data were expressed as Mean ± Standard Error of Mean (SEM). The statistical analysis was carried out according to one-way ANOVA analysis followed by Dunnett’s test compared to the control and different extracts on Graph Pad Prism software version 6.0. The level of significance was accepted at p < 0.05.
3. Results
3.1. Total Triterpenes and Sterols Contents
The total triterpenes and sterols contents of methanolic and aqueous extracts were showed in Table 1. The methanolic extracts exhibited higher levels.
3.2. Total Minimum Inhibitory Concentration and Minimum Bactericidal Concentration
MIC and MBC values of C. papaya and C. nucifera extracts on tested strains were recorded in Table 1. The Minimum Inhibitory Concentration of the extracts was greater than 2 mg/mL except for the methanolic extracts on Staphylococcus aureus which were 2 mg/mL. The MBC of the extracts on all the studied strains is greater than 2 mg/mL.
![]()
Table 1. Content of extracts in total triterpenes and sterols.
Legend: UAE: Ursolic Acid Equivalent, CE: Cholesterol Equivalent, CN M: C. nucifera methanolic extract, CN AQ: C. nucifera aqueous extract, CP M: C. papaya methanolic extract, CP AQ: C. papaya aqueous extract.
![]()
Table 2. MIC and MBC of C. nucifera and C. papaya extracts on Escherichia coli, Pseudomonas aeruginosa, Streptococcus mutans and Staphylococcus aureus.
Legend: CN M: C. nucifera methanolic extract, CN AQ: C. nucifera aqueous extract, CP M: C. papaya methanolic extract, CP AQ: C. papaya aqueous extract.
3.3. Inhibition of Extracts on Biofilm Formation
The effect of extracts from the roots of C. papaya and C. nucifera at a concentration of 100 μg/mL on the biofilm formation of Escherichia coli, Streptococcus mutans, Staphylococcus aureus, Pseudomonas aeruginosa was evaluated after 24 hours of growth. At this concentration, the extracts did not affect bacterial growth as shown in Figure 3(a), Figure 3(c), Figure 4(a), and Figure 4(c). Unlike DMSO used as a control, the extracts reduced the formation of the biofilm of the strains studied (Figure 3(b), Figure 3(d), Figure 4(b), and Figure 4(d)). The methanolic extract of C. nucifera had a better inhibition on gram-positive bacteria (52.72% inhibition of the biofilm formed by Pseudomonas aeruginosa) and the methanolic extract of C. papaya on gram-negative (66.10% of inhibition of the biofilm formed by Streptococcus mutans). The salicylic acid used as a reference substance was in general more active compared to the extracts or was not significant (inhibition of C. nucifera on P. aeruginosa).
3.4. Effect of Extract on Violacein Production
The effect of the extracts on violacein production was evaluated at the final concentration of 100 μg/mL. As shown in Figure 5(a), the viability, and growth of
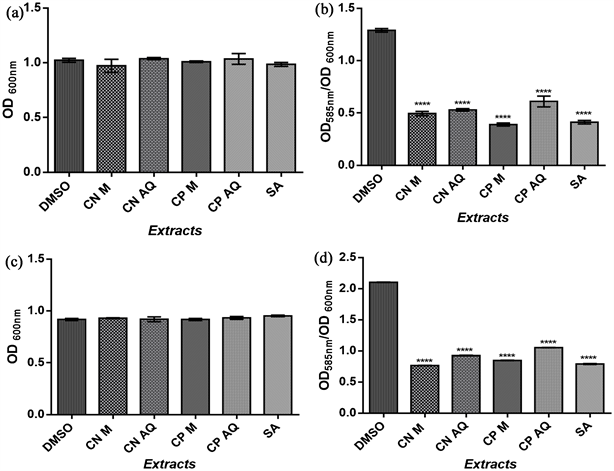
Legend: OD: optical density, CN M: C. nucifera methanolic extract, CN AQ: C. nucifera aqueous extract, CP M: C. papaya methanolic extract, CP AQ: C. papaya aqueous extract. ****p < 0.05 Extracts/Salicylic acid vs DMSO.
Figure 3. Effect of C. nucifera, C. papaya extracts, and Salicylic acid on biofilm formation of two Gram-positive bacteria. (a) Effects on E. coli growth; (b) Effects on Biofilm formed by E. coli; (c) Effects on P. aeruginosa growth; (d) Effects on Biofilm formed by P. aeruginosa.
![]() ****p < 0.05 Extracts/Salicylic acid vs DMSO.
****p < 0.05 Extracts/Salicylic acid vs DMSO.
Figure 4. Effect of C. nucifera, C. papaya extracts, and Salicylic acid on biofilm formation of two Gram-negative bacteria. (a) Effects on S. aureus growth; (b) Effects on Biofilm formed by S. aureus; (c) Effects on S. mutans growth; (d) Effects on Biofilm formed by S. mutans.
![]() ****p < 0.05 Extracts/Salicylic acid vs DMSO.
****p < 0.05 Extracts/Salicylic acid vs DMSO.
Figure 5. Effect of C. nucifera, C. papaya extracts, and Salicylic acid on QS-controlled violacein production of C. violaceum CV026.
C. violaceum CV026 were not influenced by the presence of extracts. C. violaceum strain has insufficient capacity to produce the quorum detectable by self-inducers (homoserine-lactones). Thus, the addition of homoserine-lactone in the growth medium allowed the production of violacein. Then, the reduction in the production of violacein was the consequence of the interference of the extract with the mechanisms of quorum Sensing of C. violaceum CV026 (Figure 5(b)). All extracts reduced the production of violacein with a better inhibition recorded by the methanolic extract of C. nucifera (61.42%).
3.5. Anti-Proliferative Effects on Prostate Cancer Cell Lines
The different IC50 of the methanolic extracts of C. papaya and C. nucifera corresponding to the results of the anti-proliferative activity tests are given in Table 3. The extracts exhibited anti-proliferative activity on prostate cancer cell lines LNCaP and PC-3. C. nucifera demonstrated the best activity on LNCAP, either an IC50 of 26.98 ± 2.6 µg/mL and C. papaya on PC-3 with an IC50 of 127.20 ± 5.99 µg/mL.
Figure 6 shows the viability of LNCaP and PC-3 cells according to the concentrations of each extract used.
4. Discussion
Medicinal plants have been used as a possible source of new classes of antibiotics with new modes of action and represent an alternative to treat infections caused by resistant microbes [22] . C. papaya and C. nucifera are two medicinal plants used in Burkina Faso for the management of infectious diseases and with an anti-inflammatory component such as cancer. In addition, in vitro antioxidant and
![]()
Table 3. IC50 of methanolic extracts of C. nucifera and C. papaya on the viability of LNCaP and PC-3 cells in prostate cancer.
Legend: IC50: Inhibitory concentration 50; Values are expressed as mean values ± standard deviation. n = 3 independent experiments.
![]()
Figure 6. Dose-dependent anti-proliferative activity of C. nucifera and C. papaya extracts on human LNCAP and PC-3 cell lines of Prostate Cancer Cell lines were treated for 72 h. Experiments were performed 3 times in sextuplets.
anti-inflammatory properties of extracts from these plants have been demonstrated previously [13] [23] . The same study showed that the extracts are rich in polyphenols, tannins, and flavonoids [13] . It emerges from this study that the extracts of C. nucifera and C. papaya are rich in triterpenes and sterols (Table 1). The lipophilic nature of terpenes offers cytotoxic activities against a wide range of organisms ranging from bacteria to selected organisms. They are used in herbal medicine against infections [24] .
Biofilm formation is a major problem factor in many areas, ranging from industrial corrosion and biofouling to chronic and nosocomial infections [25] . Indeed, many strains of uropathogens can form a biofilm (physical barrier against antimicrobial agents) allowing them to avoid the immune system and increase their resistance to recommended drugs [26] [27] . The results of this study showed that the extracts resulted in the formation of biofilm on the resistant strains used (Figure 3 and Figure 4). The eradication of biofilms is of great importance in the fight against infection [27] . The ability to inhibit the biofilm of E. coli by the ethanolic extract of the leaves of Carica papaya over a range of concentrations was shown in the study conducted by Hastuty [28] . Coconut water has an anti-biofilm activity of P. aeruginosa [29] . The tannins, flavonoids, and triterpenes in the extracts are known for their ability to disrupt the biofilm of bacteria by interfering with its mechanism [28] [30] . Biofilms are associated with microbial infections and their formation can be regulated by quorum sensing [30] . Because of continuing emergence and spread of multidrug-resistant bacteria, an antipathogenic strategy to combat bacterial infections through the disruption of quorum sensing controlled virulence factors has received increased attention [31] . In the present study, one of the best-known cases of quorum-sensing-regulated phenotypes, pigment production by the bio-reporter strain of C. violaceum was used to screen the four extracts for their potential for inhibiting quorum sensing (Figure 5). Quorum sensing is a recently discovered chemical communication system that enhances bacterial survival, as a group allowing resident bacteria to assume specialized roles vital for the regulation of intra- and inter-bacterial genes, and for maintaining bacterial colonies intact [31] [32] . Violacein production by C. violaceum was significantly inhibited by the extracts in the present study. The decrease in the production of violacein is therefore the consequence of interference of the extracts with the mechanisms of QS. The effect of the extracts could be due to the polyphenols they contain and in particular to the flavonoids known for their anti-QS potential [33] [34] . In general, most of the extracts showed no antibacterial activity within the limit of the maximum concentration used which was 2 mg/mL (Table 2).
This might be due to the effects of the extracts’ compounds in limited molecular target areas, which could involve only in quorum sensing signaling of the bacteria [19] [31] . Thus, our result indicates the need for a thorough examination of plants by identifying the active compounds and the mechanism of quorum sensing actions by the active compound. The methanolic extracts demonstrated a better antibacterial effect than the aqueous extracts which could be due to the difference in the contents of the phytochemical compounds highlighted. In addition, the extracts are more active against gram-positive bacteria (S. mutans, S. aureus) than gram-negative bacteria (E. coli, P. aeruginosa). The peptidoglycan layer present in gram-positive bacteria could be the target of phytochemicals contained in the extracts [35] .
Cytotoxicity was estimated using the MTT assay, which is a routine test assessing cell viability and proliferative activity through cell enzyme activity [36] . Human prostate adenocarcinoma cell lines, such as LNCaP (androgen-dependent), and PC-3 (androgen-independent) are among the most often used in vitro experiments [37] . The extracts exhibited a detrimental effect on cancer cell viability in a concentration-dependent manner, unlike the DMSO negative control which did not affect the cells (Figure 6). According to the classification of the plant screening program of the National Cancer Institute of the United States, the extract of C. nucifera has good cytotoxic potential on the viability of LNCaP cells of prostate cancer with an IC50 = 26. 98 ± 2.6 μg/mL (Table 3). Furthermore, the extracts of C. papaya and C. nucifera show a moderate effect on the PC-3 lines. According to Bayala et al. [38] , the cytotoxic effects in this study could be attributed in part to the antioxidant potency of the extracts demonstrated in the previous studies. Indeed, the cytotoxic and anti-proliferative effect of triterpenes, tannins, flavonoids, and saponins highlighted in the extracts has been proven [38] [39] .
5. Conclusion
This study demonstrated that extracts from the roots Carica papaya and Coco nucifera are remarkable antibacterial agents against the biofilm formed by Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, and Streptococcus mutans strains, and while inhibiting the signal molecules, which form the basis of the bacterial communication mechanism. Cocos nucifera extract has therefore shown a good cytotoxic effect and could be further investigated in order also to develop an anticancer phytomedicine. Those data provide scientific data and show that the folkloric uses of the roots of Carica papaya and Cocos nucifera to treat certain health problems could have a scientific basis.
Acknowledgements
The authors are thankful to the Laboratory of Applied Biochemistry and Chemistry (University Joseph KI-ZERBO); Pietro Annigoni Biomolecular Research Center (CERBA); and the Department of traditional medicine of Research Institute of Health Sciences (IRSS).
Authors’ Contributions
WLME B-K performed the tests, analyzed the results, and wrote the manuscript. VO and AB contributed to the achievement of antibiofilm and anti-quorum activity. BB guided antiproliferative test. EO contributed to the performance of antiproliferative activity. BY contributed to perform the phytochemical dosage. LB, MC and MK reviewed the final version. NO supervised the study, read and approved the final version of the manuscript.