The Clinical Association of Left Atrial Function with Left Ventricular Ejection Fraction ()
1. Introduction
The utility of left atrial (LA) volume and function for monitoring cardiovascular risk and for guiding therapy is an evolving science that may indeed prove to have a very important public health impact [1] [2] [3] [4] . The LA has been recognized as a morphophysiological barometer of left ventricular (LV) dysfunction [5] . Impaired relaxation and increased stiffness of the LV may alter LA loading conditions, resulting in changes in LA size and function [6] [7] [8] . Therefore, because in the myocardial ischemia cascade where LV diastolic dysfunction often precedes LV systolic dysfunction, the LA which fashions as an early marker of diastolic anomaly, could equally reflect a declining LV function and/or be a good predictor of potential sequelae [9] [10] [11] . We assessed this association of LA function with reduced LV systolic function among hospitalized patients.
2. Methods
We retrospectively reviewed echocardiography reports of unselected, consecutive, hospitalized patients in sinus rhythm who had 2-dimensional complete transthoracic echocardiography for various inpatient reasons. The study was approved by the Institutional Review Board. Patients who had greater than mild valvular heart disease were excluded from our analysis. In addition, echocardiograms with poor image quality were omitted.
LA volume was measured by manually tracing the endocardial contours of the LA end of LV systole (VOLmax), just before atrial contraction (VOLac), and at the end of LV diastole (VOLmin) in parasternal long axis and apical 4-chamber views [12] . From those volumetric measurements, the following parameters of LA function were calculated: 1) LA passive emptying fraction (LAPEF) = (VOLmax − VOLac) × 100%/VOLmax; 2) LA active emptying fraction = (VOLac − VOLmin) × 100%/VOLac, and left atrial kinetic energy (LAKE) = LA kinetic energy (LAKE) = 1/2 × 1.06 × (density of blood in g/cm-cubed) × LA stroke volume × A- wave velocity-squared [13] . End-left ventricular systole was defined as the frame depicting the cardiac systolic event just before separation of the mitral valve tips. End-left ventricular diastole was defined as the frame depicting the cardiac diastolic event just before complete coaptation of the mitral valve tips. Atrial contraction was defined as the frame depicting the cardiac event coinciding with the onset of the P wave. Both the atrial appendage and the pulmonary veins, when visualized, were carefully excluded from the region of interest during planimetry.
Data are expressed as mean ± standard deviation (SD) for continuous variables and frequencies for categorical variables. Differences between groups were assessed using Chi-square statistics for categorical variables and analysis of variance for continuous variables. A p value < 0.05 was considered significant. Pearson correlation coefficient (r) with a multivariate logistic model was also analyzed. Formulaic estimation of LA linear measurement and that by TTE was also assessed on a Bland Altman plot. Statistical analyses were performed using SPSS Version 13.0 statistical software (SPSS Incorporated, Chicago, Illinois). The study was approved by our Institutional Research Board and is in keeping with the ethical values delineated by the Declaration of Helsinki.
3. Results
Our sample comprised of 294 patients (18 - 95 years of age; mean ± SD 62 ± 17.4; 58.8% female) who met criteria (Table 1). LV ejection fraction (LVEF) was strongly associated with LA Passive emptying fraction (LAPEF) (p < 0.0001; Table 2 & Figure 1 and Figure 2).
![]()
Table 1. Baseline characteristics of patients in study (N = 294).
LVEF—Left Ventricular Ejection Fraction, LA—Let Atrium, AP—Antero-posterior, LA VOLmax—Left Atrial Volume at the end of ventricular systole, LA VOLac—Left Atrial Volume just before atrial contraction, LA VOLmin—Left Atrial Volume at the end of ventricular diastole, PLAX—Parasternal Long Axis View, A4C—Apical Four Chamber View, DT—Deceleration Time, LVIDd—Left Ventricular Inner Diameter in diastole, Sep.d—Septal thickness in diastole, PLVWd—Posterior Left Ventricular Wall thickness in diastole, LAPEF—Left Atrial Passive Emptying Fraction, LAAEF—Left Atrial Active Emptying Fraction, LAKE—Left Atrial Kinetic Energy
![]()
Table 2. Correlation between LVEF and LA function
LVEF—Left Ventricular Ejection Fraction, LA—Let Atrium, PLAX—Parasternal Long Axis View, A4C—Apical Four Chamber View, LA Passive EF—Left Atrial Passive Emptying Fraction, LA Active EF—Left Atrial Active Emptying Fraction, LAKE—Left Atrial Kinetic Energy.
![]()
Figure 1. Correlation of passive LAEF with LVEF on 2 D
Among patients with reduced LVEF, measurements with 2D Echo showed that LAPEF was lower (0.149 ± 0.10 vs. 0.197 ± 0.13, p = 0.023 in PLAX view and 0.172 ± 0.12 vs. 0.232 ± 0.14, p = 0.013 in A4C view) and LAKE was higher (4.69 ± 4.2 vs. 4.17 ± 3.2, p = 0.362 in PLAX view and 6.48 ± 6.3 vs. 4.57 ± 3.5, p = 0.005 in A4C view; Table 3(a)).
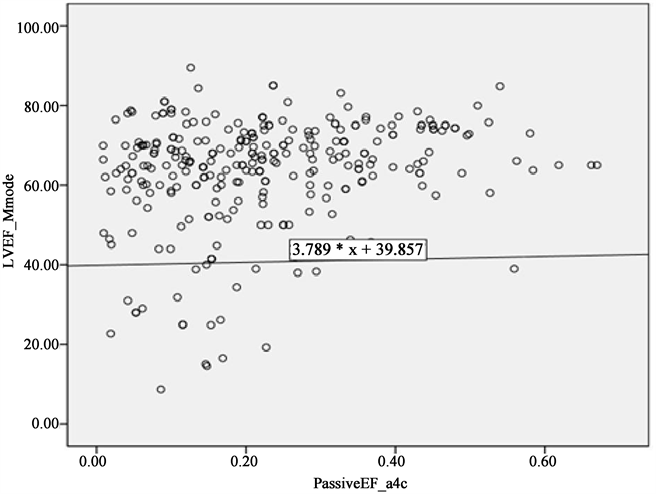
Figure2. Correlation of passive LAEF with LVEF on M Mode
(a) ![]() (b)
(b) ![]()
Table 3. (a) Association of LA function in patients with and without preserved LVEF on 2D Echo; (b) Association of LA function in patients with and without preserved LVEF on M-mode.
We noted similar results with M-Mode measurement among those with reduced LVEF. Reduced LAPEF in PLAX (0.142 ± 0.09 vs. 0.197 ± 0.13, p = 0.010) and in A4C views (0.159 ± 0.11 vs. 0.233 ± 0.14, p = 0.002) and increased LAKE in PLAX (4.59 ± 4.3 vs. 4.19 ± 3.2, p = 0.504) and A4C views (6.81 ± 6.4 vs. 4.54 ± 3.5, p = 0.001; Table 3(b)) remained equally associated.
4. Discussion
Our study is the first to show a comprehensive association between reduced LVEF and LA function. LAPEF is an active energy-driven process that appears to be one of the first mechanisms to be affected when LV diastolic dysfunction sets in. Indeed, the LA plays a pivotal role in filling of the LV. It serves as a reservoir of expansion during LV systole, as a filling conduit during LV diastole and actively contributing to LV volume with an “atrial kick” during its active phase, when sinus rhythm is present, during late LV diastole [4] . This active phase has an important role in the compensation for decreased LV compliance in patients with heart failure. We know that cardiac output decreases by 15% - 20% in patients who are in atrial fibrillation or are only paced in the ventricle [14] . This is not only of importance from a pathophysiological point of view, but LA function is also an independent determinant of prognosis in patients with heart failure [15] . In this study, we were able to demonstrate that LAKE was higher in patients with reduced LVEF as compared to patients with preserved LVEF. Tripokiadis et al. [13] also demonstrated that left atrial pump function increases during heart failure and so compensates for the decrease in left ventricular function. Hence, it‟s important to assess the degree of LA modifiability after medical treatment because by doing so, individual treatment can be optimized not only by taking into account the clinical status, renal status, ventricular remodelling and neurohormonal status (BNP), but also an atrial contribution to left ventricular filling.
There are several possible mechanisms by which myocardial ischemia leads to abnormalities of LV filling early in diastole and thus to a diminution of LA passive functional reserve [2] [5] [16] [17] [18] :
1) Myocardial relaxation is an exquisitely energy-dependent process and highly sensitive to the effects of ischemia, because adenosine triphosphate hydrolysis is needed to release tightly bound actin-myosin bonds and for calcium reuptake into the sarcoplasmic reticulum; and 2) subendocardial longitudinal fibers are particularly sensitive to ischemia. This leads to uncoordinated or asynchronous relaxation between circumferential and longitudinal fibers in ischemic areas, as well as a reduction in the longitudinal contractile function that may not be initially detectable but which could lead to diminished ventricular suction. We found that LAPEF was lower in the patients with reduced LVEF and is directly correlated with LVEF. Thus, echocardiographic estimation of LA function can reflect a declining LV function and/or be a good predictor of potential sequelae. This study shows that LA remodeling is more severe in patients with reduced LVEF as compared to preserved LVEF and this may be of significant clinical relevance regarding the morbidity and mortality of these two conditions. Further studies are needed to evaluate the natural history of LA remodeling in these diseases, and whether regression of LA size translates into improved cardiovascular outcomes.
Magnetic resonance imaging has been a gold standard for assessing LA function [19] but its time consuming, and in this current economic scenario it’s not a cost efficient tool. Our study shows that 2D Echocardiography can be a very useful modality to assess LA function and at the same time is less time consuming, requires fewer resources and is cost effective. Emerging echocardiographic modalities like 3D Echocardiography (3DE) and Speckle Tracking Echocardiography (STE) have shown promise in quantifying LA volume and function and appears to be more accurate but the data regarding their feasibility and clinical utility in routine clinical evaluation is lacking. Meanwhile, 2D echocardiographic assessment of LA function should be part of the complete echocardiographic examination and should not be neglected, as it had been for some time.
Several study limitations should be considered when evaluating the clinical implications of our findings. Our cohort, being in patients who were imaged for various presentations may or may not have had a definite form of heart disease and may, therefore, not perhaps be the most ideal to test such a hypothesis of reduced LA function with impaired LV function. However, LA function speaks volumes and serves to answer the clinical question irrespective of whether patients were “sick”, “less sick” or healthy particularly when our assessment was directed to answer the clinical question specifically in a hospitalized setting. Because the LA is a thin-walled structure, border detection and thus, precise manual tracing can sometimes be a challenge and may especially be considerably influenced when images are poor. We, however, excluded those with poor image quality, accounting for the small final study sample.
5. Conclusion
LA function appears to be associated with LVEF, be it on 2D Echo or M-mode. This could be particularly important when risk-stratifying hospitalized patients. These findings, when used among heart failure patients, could help in the focused management and direction of treatment, especially in the higher-risk cohort of heart failure patients with concomitant LA dysfunction.