Positive and Negative Environmental Effect of Using Zinc Oxide Nanoparticles on Wheat under Drought Stress ()
1. Introduction
Nanoscience is a development concept in agriculture field. In recent years, the rapid evolution of nanotechnology has made it possible to synthesize engineered nanoparticles of various types, sizes and morphologies [1]. NPs have unique properties, such as surface area, pore size, particle morphology, and reactivity. NPs can be used as nano-fertilizers, nano-insecticides, and herbicides, and can use to increase crop productivity, control excessive use of chemical fertilizers, and improve survivability against biological stress. They regulate plant development and increase metabolic activity [2] [3]. NPs may have a positive or negative effect on plant growth species according to their type and concentration used.
Rajput et al. [4] and Xiong et al. [5] studied the bioaccumulation of nanoparticle on plants, and it was found that long-term exposure influences the food chain.
Gottschalk et al. [6] stated that more NPs are present in soil than in water and air. The impact of NPs on agriculture, the atmosphere, or society can be either gradual or destructive. Several studies have shown that nanoparticle is able to absorb and accumulate in crops tissues [7] [8]. This accumulation will occur in the form of NPs or metal ions in their original form. However, it may be different due to different characteristics. So, the accumulation of NPs led to change in the physiological processes of plants by producing hydroxyl free radicals to change the content of protein, lipid, and nucleic acid [9] [10] [11]. However, negative impact of NPs on human health has been uncertain [12].
Water Scarcity is one of the biggest serious stresses that limit growth of plant. Due to water stress, many changes have been observed in plants as mineral nutrient balance, biologically active compounds [13].
Microelement fertilizers enhanced the resistance of plants to environmental stresses such as water shortage. Zinc is an important element for plant metabolism, water balance and stoma regulation. It regulates various enzymatic functions and is necessary for the biochemical reactions that lead to the chlorophyll synthesis [14]. Presently, using of nanoparticles as ZnO-NPs has expanded [15]. The use of micronutrient fertilizers in the shape of nanoparticles is an appropriate procedure that can gradually and controllably release the required nutrients, which is essential to alleviate the problem of fertilizer pollution. Due to the high specific surface area, highly active surface, unique size and shape, and chemical, physical, biological, and catalytic properties, NPs can regulate the chemical and biological activities of cells. Also, ZnO-NPs possess excellent electrical properties [16]. Zinc oxide (ZnO) nanoparticles showed positive effects on peanuts, glycine max, wheat, and onion at low concentrations [17] [18]. When different rates of ZnO NPs are applied to plants, such as cucumber, alfalfa, and [19], only cucumber germination will increase. In Cymopsistetragonoloba, ZnO NPs increased plant biomass, plant growth, chlorophyll, and protein content [20]. Supplementation of ZnO NPs to MS medium can promote embryogenesis, seedling regeneration, plant development and some enzymes (proline, superoxide dismutase, catalase, and peroxidase), thereby alleviating biotic stress [21]. The presence of ZnO NPs enhances antioxidant properties, photosynthetic efficiency, and proline accumulation, resulting in plant stability [22]. Therefore, this study aimed to evaluate the positive and negative effects of ZnO NPs on the growth of wheat plants under water scarcity.
2. Material and Methods
2.1. Biosynthetic and Characterization of Zinc Oxide Nanoparticles
Extraction preparation was done according to Geetha et al. [23] and Jabeen et al. [24] with some modifications as shown in Diagram 1. The surface area was determined by BET analysis of the produced nanoparticles using a Quantachrome analyzer (Nova 2000 series, USA) using an N2 vapor adsorption experiment. Using a field emission scanning electron microscope (FE-SEM) equipped with a microscope (JEOL JSM-6390) and a high-resolution transmission electron microscope (HR-TEM) (JEM-2100) to study the morphology of the prepared product under an acceleration voltage of 200 kV.
2.2. Field Experiment
A Field experiment was carried out during successive season (2019/2020) at Experimental Farm, Agricultural Genetic Engineering Research Institute, Agricultural Research Center, Giza, Egypt, to evaluate the positive and negative impact of ZnO nanoparticles on wheat plant growth under drought stress. The field trial consisted of 4 square meters “2 × 2” meters regional sample plots, with 3 replicates for each treatment and control. The experimental layout is designed based on random complete blocks. Some soil properties are shown in Table 1. Soil was
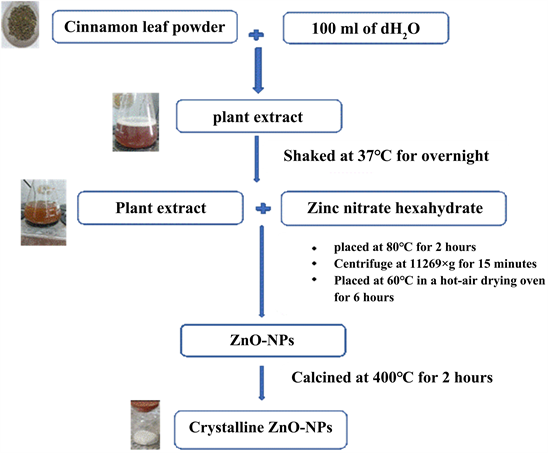
Diagram 1. Preparation of ZnO NPs.
![]()
Table 1. Physical and chemical properties of soil.
fertilized with the recommended levels of nitrogen, phosphorous and potassium. NP-Zn O was applied either by seed-soaking, foliar spraying (after 2, 4, 6 and 8 weeks from sowing), or both together applications. The dose of ZnO NPs was 100 mg/l [25]. Normal Zn and zinc nano particles (Zn-NPs) were applied in this experiment. Five Treatments: Without ZnO as control, normal Zno as foliar spraying, Zno-NP as seed soaking, Zno-NP as foliar spraying, soaking, and spraying together. All treatments were done under drought stress or no stress. Water scarcity is achieved by cutting off water after the milking phase until the end of the season.
2.3. Analytical Methods
Some properties of soil were measured according to Page et al. [26]. Regarding the plant analysis, leave chlorophyll was measured by method of Lichtenthaler [27]. Proline was determined according to Bates et al. [28]. Folin-Ciocalteu phenol reagent was used for determined total phenol according to Singleton and Rossi [29]. Hormones were extracted and identified according to Wurst et al. [30] by using HPLC Agilent 1250. Catalase, Peroxidase and Superoxide Dismutase activity were determined in leaves (Aebi [31]; Polle et al. [32]; Giannopolitis and Ries [33]). Dried plant materials were digested according to the procedure of Chapman and Pratt [34]. Zinc was measured to Jackson [35].
2.4. Statistical Analysis
Result obtained was exposed to the proper statistical analysis of complete randomized design [36]. Means obtained were differentiated by Duncan’s new multiple ranges test [37].
3. Results and Discussion
3.1. BET Analysis and X-Ray Diffraction (XRD)
XRD analysis
The X-ray diffraction pattern of the as-prepared ZnO nanoparticles is shown in Figure 1(c). Diffraction peaks at 2-theta at 31.71˚, 34.37˚, and 36.2˚ correspond to (100), (002), and (101) planes, respectively, and confirm the formation of ZnO with a hexagonal structure. All peaks are well matched to JCPDF card number: 01-076-0704. To calculate the average crystallite size of the synthesized ZnO nanoparticles, we used the Scherrer formula (1) [38],
(1)
![]()
Figure 1. (a) FESEM, (b) HRTEM, and (c) XRD diffraction pattern of the nanostructure ZnO prepared by cinnamon extract.
where D is the average crystallite size, K is a constant equal to 0.94, λ is the wavelength of the X-ray (0.154 nm), b is the full-width peak half maximum (in radians), and θ is the Wasragg angle (degrees). We have calculated the crystallite size of the diffraction peaks (100), (002) and (101) using the Scherrer equation and found that the average crystallite size value was about 18 nm. The lattice parameters were found to be a = 3.25 Å, b = 3.25 Å, c = 5.21 Å, and the lattice angles were a = 90˚, b = 90˚ and g = 120˚, respectively. The space group of the as-prepared ZnO nanoparticles is 186: P63 mc. The intensities and positions of the diffraction peaks are consistent with the reported values [39].
The morphology of the thus prepared products was examined using a field emission scanning electron microscope (FE-SEM) equipped with a microscope (JEOL JSM-6390) and a high-resolution transmission electron microscope (HR-TEM) (JEM-2100). At an accelerating voltage of 200 kV, the resulting nanoparticles were single-phase with no impurity spikes. The obtained results of BET analysis showed that the produced nanomaterial has surface area of 12.35 m2·g−1, indicating that the average particle size of the produced nanomaterial within the nanoscale. Figure 1(b) represents the high-resolution images TEM of the prepared nanostructure zinc oxide. The high magnification image reveals that the uniform size of the prepared oxide with a range of 2.4 to 3.7 nm. Furthermore, the nano size ZnO morphology was studied by SEM as seen in Figure 1(a). Moreover, the SEM images of the nanoparticles ZnO at high magnification show that the morphology was undefined spherical shape with rough surface.
3.2. Effect of ZN-NPs on Yield
Water scarcity reduced the wheat yield of grain and shoot. The use of different forms of ZnO improved the grain and shoot wheat yield. Results of grain and shoot yield were recorded in Figure 2. The lowest value yield was noticed in plants that were untreated with different forms of Zno under drought stress or no stress. The maximum decrease under drought stress, the maximum decrease for grain and shoot yield reached 12.50% and 8.41%, respectively compared to normal ZnO. On the contrary, data showed that application of Zn-NPs under investigation (seed soaking, foliar spraying, and seed soaking + foliar spraying) increased mightily grain and shoot yield under no stress or drought stress. For grain yield, these increases reached 1.04-, 1.11-, and 1.21-fold, respectively that control without ZnO under no stress, while reached 1.26-, 1.38-, and 1.45-fold, respectively that control without ZnO under drought stress. For shoot yield, these increases reached 1.06-, 1.14-, and 1.16-fold, respectively that control without ZnO under no stress, while reached 1.13-, 1.15-, and 1.18-fold, respectively that control without ZnO under drought stress. Regarding the natural ZnO impact, it was noticed that there was an increase in the productivity of the grain and straw crop compared to not using ZnO. It is worth noting that the favorable effect of zinc on yield was evident under the influence of drought than no stress. Generally, under drought stress, plants have adapted to osmotic and
![]()
Figure 2. Effect of drought and of ZnO-NPs on wheat yield.
ionic stress. These data corresponded with data of Adrees et al. [40], who reported that spraying of ZnO NPs was more effective in a crop under water shortage while reducing the chance of NPs moving to other environmental compartments. Dimkpa et al. [41] stated that ZnO NP corrected drought-stressed sorghum plants and improved plant growth and plant’s absorption of total nitrogen and potassium. In addition, foliar spraying of zinc oxide nanoparticles improved the water deficit tolerance of eggplants in saline soil. These positive effects mainly come from improved absorption of macro- and micronutrients, increased relative water content (RWC), reduced cell membrane damage, and improved anatomical characteristics of eggplant leaves and stems [42].
Zinc affects the water conditions of plants as it alters stomata conductivity and transpiration. Zinc concentration affects the activity of zinc-dependent enzymes such as carbonic anhydrase, which regulates the CO2 sensing pathway and is associated with drought tolerance [43]. SOD and CAT are involved in antioxidant defense under water scarcity [44].
Karim and others [45] stated that under well water conditions, Zn spraying had no effect on wheat grain yield, but increased grain yield and grain Zn concentration under drought conditions. Sultana et al. [46] found that spraying leaves with zinc offsets the detrimental effects of water scarcity on wheat yield. Due to their small size, structural properties, and large surface area and volume, so nanoparticles can promote plant growth under stress [47]. 20% - 25% increase in wheat yield from seeds treated with metal nanoparticles [48].
3.3. Effect of ZN-NPs on Chlorophyll of Leaves Wheat
Results of leaves chlorophyll were recorded in Figure 3. The maximum decrease
![]()
Figure 3. Effect of drought and of ZnO-NPs on cholorophyll of wheat leaves.
of chlorophyll was noticed under drought stress, this decrease reached 38.9% compared to normal ZnO. On the contrary, data showed that application of Zn-NPs under investigation (seed soaking, foliar spraying, and seed soaking + foliar spraying) increased mightily leaves chlorophyll under no stress or drought stress. These increases reached 1.10-, 1.36-, and 1.57-fold, respectively that control without ZnO under no stress, while reached 1.69-, 1.98-, and 2.16-fold, respectively that control without ZnO under drought stress. It is important to note that zinc nanoparticles had an effective role in increasing chlorophyll compared to natural zinc. In general, severe water stress inhibits the efficiency of photosynthesis which is associated with the decomposition of chlorophyll [49]. Zinc addition participates in chlorophyll biosynthesis by protecting sulfhydryl groups, chloroplast development and some functions [50]. Also, ZnO-NPs increase organic carbon of soil, which is released from root exudates treated with ZnO-NPs, which may increase the photosynthetic rate [51].
3.4. Effect of ZN-NPs on Enzyme Activities of Wheat Leaves
Results of catalase, peroxidase and superoxide dismutase were shown in Figure 4 and Figure 5. Data showed that using of Zn-NPs (seed soaking, foliar spraying, and seed soaking + foliar spraying) increased enzyme activity under no stress or drought stress. For catalase, these increases reached 1.04-, 1.06-, and 1.08-fold, respectively that control without ZnO under no stress, while reached 1.05-, 1.15-, and 1.20-fold, respectively that control without ZnO under drought stress. For peroxidase, these increases reached 1.04-, 1.06-, and 1.08-fold, respectively that control without ZnO under no stress, while reached to 1.11-, 1.22-, and 1.30-fold, respectively that control without ZnO under drought stress.
![]()
Figure 4. Effect of drought and of ZnO-NPs on catalase and peroxidase enzymes.
![]()
Figure 5. Effect of drought and of ZnO-NPs on dismutase activity.
At the same trend there was an increase in dismutase activity, these increases reached 1.01-, 1.02-, and 1.05-fold, respectively that control without ZnO under no stress, while reached to 1.03-, 1.14-, and 1.18-fold, respectively that control without ZnO under drought stress. It is important to mention that nano-zinc had an effective role in increasing enzyme activities compared to natural zinc. In addition, one of the important roles of zinc is to increase antioxidant levels in plant tissues, as it acts as a cofactor for enzymes such as superoxide dismutase, peroxidase, and catalase. Zinc plays an important role in protecting and maintaining the stability of cell membrane structures [52]. These data are consistent with those of Mohsenzadeh and Moosavian [53], who reported that foliar sprays of zinc sulfate and nano-zinc oxide had a positive effect on the antioxidant enzyme activities of rosemary. Taran et al. [54] stated that zinc nanoparticles reduced the adverse effects of drought on wheat seedlings by increasing the activity of antioxidant enzymes. Upadhyaya et al. [55], reported that exposure to different concentrations of ZnO nanoparticles increased antioxidant enzymes in rice plants.
3.5. Effect of ZN-NPs on Plant Hormones of Wheat Leaves
Data of plant hormone (indole acetic acid, gibberellic acid, cytokinin, and Abscisic acid) was listed in Figure 6. The results showed an increase of either indole and cytokinin hormones in wheat leaves that were exposed to normal or nano zinc under natural conditions or drought stress, and the effect of ZnO NPs under study (seed soaking, foliar spraying, and seed soaking + foliar spraying) was more than that of natural zinc. For indole acetic acid, the increase reached 1.02-, 1.23-, and 1.43-fold, respectively that normal ZnO under no stress, while reached 1.16-, 1.47-, and 1.56-fold, respectively that normal ZnO under drought stress. For cytokinin, the increase reached 1.03-, 1.15-, and 1.21-fold, respectively that normal ZnO under no stress, while reached 1.06-, 1.26-, and 1.33-fold, respectively that normal ZnO under drought stress. On the contrary, the behavior of abscisic acid differed, as its concentration increased significantly in the plant exposed to drought stress compared to the plant that was not exposed to drought as a control, this increase reached 50.4%. These data are confirmed by Semida et al. [56], who indicated that the lack of water and the increase of salts in the soil led to the accumulation of abscisic acid in plant, which leads to osmotic stress. Dhalim and Ajeel [57] stated that foliar sprays of zinc sulfate, zinc oxide, and nanozinc at 100 ppm in sunflower, increased auxin and gibberellin level. Also, its prominent role in gene expression related to abiotic stress, zinc also plays important role in controlling the production of the IAA hormone, which is important for cell elongation of plant [58].
3.6. Effect of ZN-NPs on Proline of Wheat Leaves
Data of proline was shown in Figure 7. Data showed that using of Zn-NPs (seed soaking, foliar spraying, and seed soaking + foliar spraying) increased proline content under no stress or drought stress. For proline, these increases reached 1.30-, 1.63-, and 1.81-fold, respectively that control without ZnO under no stress, while reached 1.33-, 2.05-, and 2.58-fold, respectively that control without
![]()
Figure 6. Effect of drought and of ZnO-NPs on plant hormone of wheat.
![]()
Figure 7. Effect of drought and of ZnO-NPs on proline.
ZnO under drought stress. Generally, proline and phenol were increased under drought stress. These data were corroborated by data from Mohsenzadeh and Moosavian [53], who reported that foliar sprays of zinc sulfate and nano-zinc oxide increased proline content. As the most stable amino acid, proline is ubiquitous in many plants and naturally accumulates in large amounts under environmental stress. In addition, due to its role as an osmotic agent, proline stabilizes microcellular structures such as cell membranes and proteins and destroys free radicals under stress. Regarding stress, plants are compatible with the production of metabolites such as amino acids, antioxidants, and hormones to counteract the effects of stress and maintain growth [59].
3.7. Effect of ZN-NPs on Phenol of Wheat Leaves
Data of proline was shown in Figure 8. Data showed that using of Zn-NPs (seed soaking, foliar spraying, and seed soaking + foliar spraying) increased phenol content under no stress or drought stress. These increases reached 1.03-, 1.14-, and 1.19-fold, respectively that control without ZnO under no stress, while reached 1.13-, 1.28-, and 1.33-fold, respectively that control without ZnO under drought stress. The increase in total phenols may be to the effect of phenolic antioxidants. The role of zinc in the production of phenolic compounds from carbon in the shikimic acid cycle may be one of the reasons for this increase [60].
3.8. Effect of ZN-NPs on Macronutrient of Leaf Wheat
Results of macronutrient were recorded in Figure 9. The minimal macronutrient (N, P, and K) values were noticed in plants that were untreated with different forms of Zno under drought stress or no stress. The maximum decrease under drought stress, the maximum decrease for N, P, and K reached 7.69 and 8.00, and 8.00%, respectively compared to normal ZnO. Data showed that applied of Zn-NPs under investigation (seed soaking, foliar spraying, and seed
![]()
Figure 8. Effect of drought and of ZnO-NPs on phenol content.
![]()
Figure 9. Effect of drought and of ZnO-NPs on macronutrient of wheat.
soaking + foliar spraying) increased N, P, and K under no stress or drought stress. For N, these increases reached 1.03-, 1.18-, and 1.24-fold, respectively that control without ZnO under normal condition, while reached 1.21-, 1.46-, and 1.71-fold, respectively that control without ZnO under drought stress. For P, these increases reached 1.10-, 1.15-, and 1.35-fold, respectively that control without ZnO under no stress, while reached 1.17-, 1.35-, and 1.48-fold, respectively that control without ZnO under drought stress. For K, these increases reached to 1.04-, 1.07-, and 1.15-fold, respectively that control without ZnO under no stress, while reached 1.13-, 1.17-, and 1.22-fold, respectively that control without ZnO under drought stress. The effect of natural ZnO, was observed that there was an increase in the productivity of the macronutrients compared to control under stress. Overall, the observed data suggest that the role of ZnO NPs is not only to improve plant growth and physiological parameters, but also to enhance nutrient uptake and plant nutrient levels. In this regard, the use of Zn in the form of ZnO NPs helps to mitigate the negative effects of stress conditions and improve the nutritional quality of plants. This desirable performance can be attributed to the important role of zinc in increasing protein synthesis, membrane function, and cell elongation, and stimulating plant roots to actively exchange cations, thereby helping plants absorb more nutrients [61].
3.9. Effect of ZN-NPs on Accumulation Zn of Wheat
Results of zinc content were recorded in Figure 10. The lowest content of zinc was shown in shoot and grain plants that were untreated with different
![]()
Figure 10. Effect of drought and of ZnO-NPs on zinc content of wheat plant.
forms of ZnO under no stress or drought stress. Data showed that application of Zn-NPs under investigation (seed soaking, foliar spraying, and seed soaking + foliar spraying) increased zinc accumulation under no stress or drought stress compared normal zinc oxid. These data are confirmed with data of Rameshradd et al. [62] they stated that compared with ZnSO4 treatment, ZnO nano-treated plant samples had higher zinc content in leaves and seeds. ZnO nanoparticles have a tiny size and increment absorption, translocation, and accumulation in a more dynamic manner than normal metal forms. Compared with other metal oxide nanoparticles, ZnO nanoparticle is low toxic, relatively inexpensive, and biocompatible, because zinc does not interact with most pharmacologically active molecules [63].
4. Conclusion
Zinc has a positive effect on plant growth, and this effect is strongly purified if zinc is present in the nanoform compared to the natural form. Nevertheless, it is necessary to pay attention to the extent of the accumulation of zinc particles in the different parts of the plant and not to exceed the permissible concentration in the plant which may have different environmental effects. Therefore, further studies on these environmental effects are necessary.