Salivary Active MMP-2 of Breast Cancer Patients Is Inhibited by Guava Leaves PBS Extract ()
1. Introduction
Matrixmetalloproteinases, family of enzymes, are extracellular matrix remodeling proteases whose activity has been implicated in a number of important normal and pathological processes [1]. Growth of tumor progression and metastasis as well as angiogenesis is associated with these events [2]. MMP-2, also called gelatinase-A, is a 72 Kd protein present in chromosome 16 in humans. MMP-2 contributes to cancer cell migration [3] [4], Cartoons 1(a) & Cartoons 1(b). MMPs are the key to normal development as well as the pathology of inflammatory diseases and cancer [2]. The inhibitors of MMP-2 activity [like
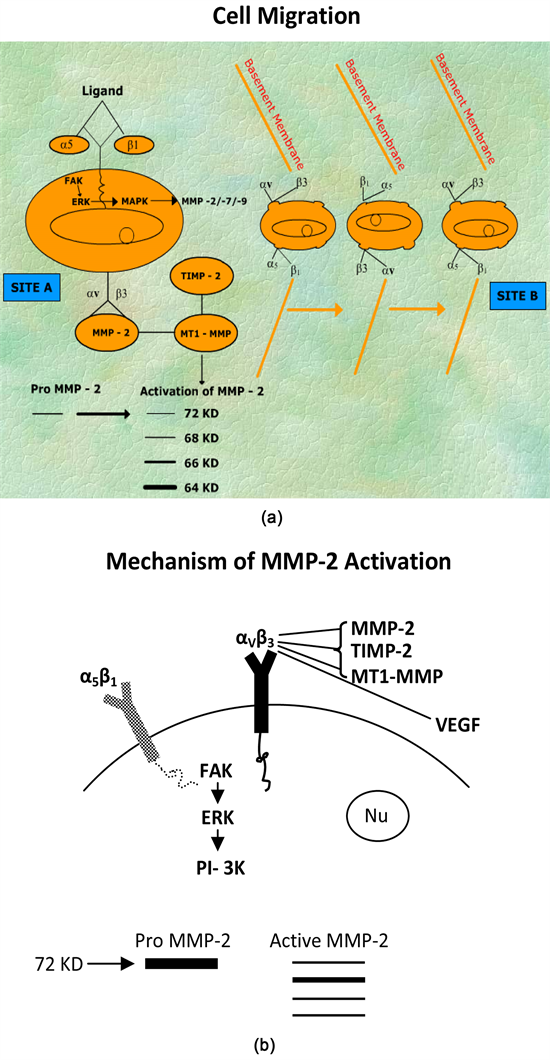
Cartoons 1. (a) Cell migration; (b) Mechanism of MMP-2 activation.
TIMP-2] are very important in maintaining the normal activity of MMP-2 and have an important role in the management of cancer [5] [6] [7]. MMP-2 has attracted attention by its roles in tumor invasion and metastasis. It was reported [8] that breast cancer patients’ saliva contains active MMP-2. Taking breast cancer patients’ (female) saliva as the source of active MMP-2 it was found that guava leaves extract can reduce the activity of MMP-2 in appreciable level [9] [10]. The chemical nature of the MMP-2 inhibitor in guava leaves PBS extract is yet to be established. The anticancer effect of guava leaves extracts was studied in details [9]. The ethanol extract of Psidiumguajava leaves extract has potential in the treatment of colorectal cancer through the angiogenesis inhibition [9]. ProMMP-2 is not readily activated by general proteinases. The main activation of MMP-2 takes place on the cell surface, mediated by MT1-MMP [11] [12]. This MT1-MMP-TIMP-2-proMMP-2 complex is then presented to an adjacent free MT1-MMP for activation. Clustering of MT1-MMP on cell surface through interactions of the hemopexin domain facilitates the activation process [11] [12].
Previous studies have demonstrated that MMP-2 and MMP-9 are important prognostic factors for various cancers [13]. The prognostic value of MMP-2/9 in Breast Cancer has been investigated [8] [13].
In this study, we are interested to see whether the PBS extract of guava leaves has any specific effects on the activity of salivary active MMP-2 of breast cancer patients.
2. Materials and Methods
2.1. Materials
The materials for SDS-PAGE was purchased from Sigma, USA. The monoclonal antibodies of MMP-2 and TIMP-2 were purchased from Santa Cruz, USA and the Pronase-K was purchased from G-Biosciences, USA.
2.2. Methods
2.2.1. Substrate Gel Electrophoresis (Zymography)
Equal amount (100 µg) of proteins from saliva (determined by Lowry’s method) run in 8% SDS-PAGE impregnated with 0.1% Gelatin. The gel was run at 25 mA using Tris/Glycine/SDS buffer (pH 8.3). The gel was washed in 2.5% Triton-X for 15 mins and then incubated in buffer A (NaCl 0.2 M, CaCl2 4.5 mM, Tris 50 mM, pH 7.4) overnight at 37˚C. The gel was stained with Coomassie Brilliant Blue to develop the zymogram [8].
2.2.2. ELISA (Enzyme-Linked Immunosorbent Assay)
To assay salivary MMP-2, TIMP-2, 50 µg of salivary proteins were used to develop ELISA using their respective monoclonal antibodies followed by 2nd antibody coupled to horseradish peroxidase (HRP). OD was taken at 450 nM [8].
2.2.3. Immunoblot Development
200 µg of salivary proteins were run on 8% SDS-PAGE. Proteins were transferred onto nitrocellulose membrane and immunoblots were developed using monoclonal antibody against MMP-2 (Santa Cruz, USA) followed by alkaline phosphatase coupled 2nd antibody. The color was developed using NBT/BCIP [8].
3. Results
Figure 1 (lanes 1-4) shows that female breast cancer patients express appreciable amount of active MMP-2 in their saliva [8]. The lane 2 of Figure 1 shows huge amount of MMP activity. The immunoblot which confirms that the MMPs secreted in breast cancer patients’ saliva are activated MMP-2 [8]. MMP-2 activity of breast cancer patients’ saliva increases stage wise has also been reported [8]. As controls, several samples of saliva of non breast cancer patients were taken (Figure 2(a), lanes 1-10). Several samples of non cancer patients (Figure 2(b), lanes 1-20) and normal female individuals (Figure 2(c), lanes 1-5) were also taken which does not show any activated (active form of) MMP-2. There are appreciable differences in the expression of active MMP-2 in female breast cancer patients and the non breast cancer patients and other controls. Cartoons 1(a) & Cartoons 1(b) show that cell surface integrin receptors alpha5beta1 (α5β1) and alfaVbeta3 (αVβ3) and MT1-MMP have important roles to play in the cellular migration and activation of MMP-2. Figure 3 shows that when guava leaves extract was added to the saliva of breast cancer patients [25 µl of breast cancer patient’s saliva and 20 µl of PBS extract of guava leaves incubated for 30 mins at 37˚C], the MMP-2 activity was appreciably reduced compared to controls, indicating presence of possible inhibitor(s) of active MMP-2 in the guava leaves PBS (x1, pH 7.4) extract [10]. Figure 4 shows that when PBS extract of guava leaves was treated with Pronase-K (100 µg/ml), the MMP-2 activity was coming back
![]()
Figure 1. Zymogram developed in four different breast cancer patients’ saliva (lanes 1-4). Lane 2 showing excess MMP activity. Methods and Materials are in the text.
![]() (a)
(a) ![]() (b)
(b)![]() (c)
(c)
Figure 2. Zymogram developed with (a) (lanes 1-10)—non breast cancer patients’ saliva, (b) (lanes 1-20)—with non cancer patients’ saliva and (c) (lanes 1-5)—normal females’ (ages 35 - 75 years) saliva as Controls. Methods and Materials are in the text.
![]()
Figure 3. Zymogram developed to see the inhibition of the expression of activated MMP-2 in three (3) (set) of the breast cancer patients. 1—Control and 2—Experimental. Methods and Materials are in the text.
![]()
Figure 4. Zymogram developed after Pronase-K treatment. 1—Control, 2—20 µl of guava leaves extract mixed with breast cancer patient’s saliva 25 µl, 3—with Pronase-K treatment (100 µg/ml).
probably indicating that the inhibitor(s) present in the PBS extract of guava leaves may be protein in nature. The guava leaves PBS extract (only) shows no gelatinase activity (band) (Figure 5). When reacted with the mouse/human TIMP-2 monoclonal antibody (Santa Cruz, USA) the ELISA of guava leaves PBS extract does not show any significant OD indicating that the inhibitor of active MMP-2 perhaps is not TIMP-2 (the known MMP-2 inhibitor) [10].
The inhibitor like molecule present in the PBS(x1) extract of Guava Leaves may be inhibitors of Zn 2+ at the active centre of MMP-2 but it may be worked out in the near future.
![]()
Figure 5. Zymogram of guava leaves extract only. Methods and Materials are described in the text.
![]()
Figure 6. Zymogram to show that the PBS extract of bamboo (Bambusabalcooa) leaf expresses activated MMP-2 like molecule. Lane-1, 50 µl of the bamboo leaves PBS extract, lane-2, only PBS.
Interesting observation is that, active MMP-2 like molecule (probably) was observed in bamboo leaves PBS extract showing very positive results in ELISA and immunoblot with MMP-2 monoclonal antibody [14] which was also completely inhibited by guava leaves PBS extract [10].
4. Discussion
The saliva of breast cancer patients contains activated MMP-2. The immunoblot clearly demonstrates that the expressed gelatinase-A/MMP-2 is reacting positively with MMP-2 monoclonal antibody. The inhibitor(s) of MMP-2 (to be identified) is very important for the research as well as for medical use. Different laboratories in the whole world are searching for new type of MMPs inhibitors from plant origin or synthetic inhibitors. In search of such MMP-2 modulator when guava leaves were extracted with PBS (pH 7.4) and reacted with breast cancer patients’ saliva the active MMP-2 was inhibited appreciably which may indicate presence of some inhibitor(s) of active MMP-2 of breast cancer patients saliva. To find out the nature of the inhibitor of MMP-2, the guava leaves extract was treated with Pronase-K (100 µg/ml), the activity was coming back (Figure 4) perhaps indicating that the inhibitor may be protein in nature. The Guava leaves PBS extract does not show any gelatinase activity (band) (Figure 5). ELISA was done of the guava leaves PBS extract with human/mouse TIMP-2 monoclonal antibody, but it was found that guava leaves PBS extract probably does not contain any TIMP-2 like molecule [10]. The immunoprecipitate also confirmed that breast cancer patients’ saliva has activated MMP-2 products [8].
Interestingly, the PBS extract of bamboo leaves express very strong activated MMP-2 like molecule and its product (Figure 6) which gives positive results in ELISA and immunoblot using human/mouse monoclonal MMP-2 antibody [14]. This plant derived probably gelatinase-A/MMP-2 activity is also completely inhibited by the PBS extract of guava leaves [10].
5. Conclusion
Guava leaves PBS extract (1x) may contain one or more inhibitors of activated MMP-2. This inhibits mammalian activated MMP-2 as well as plant activated MMP-2 like molecule. The activated MMP-2 inhibitor(s), if identified, will be very important in the management of breast cancer in future.
Acknowledgements
We are grateful to our university’s vice chancellor and pro chancellor for allowing us to use the laboratory and chemicals.
Contribution of Authors
Amitava Chatterjee is the Principal Investigator, Syandan Sinha Ray and Subhajit Mondal did the experiments, and Ramanuj Mukherjee is the Professor in Surgical Oncology, R. G. Kar Medical College involved in this Project work.