1. Introduction
Wildfire flashovers are forms of sudden fire eruptions hosted primarily by Mediterranean climate wildlands [1] [2] and have been previously reported by firefighters who were overwhelmed by their occurrences in wildfires [3] [4]. They are also referred to by the scientific community as Accelerated Forest Fires (AFFs) [5], eruptive fires [6], or generalized blaze fires (GBFs) [4]. Flashovers in wildfires [7], occur when an accumulated mixture of plants emissions composed of biogenic volatile organic compounds (BVOCs) and their degradation products, combusts rapidly. Literature provides records about the emission factors (EF) and emission ratios (ER) of BVOCs [8] [9] from Mediterranean forests in wildfire events because of their role in bio-sphere-atmosphere interactions [10] and global warming [11]. However, as flashovers are more commonly witnessed by firefighters, it is interesting to analyse the role of BVOCs and their degradation products, as a favouring factor.
Bicyclic monoterpenes such as α-pinene possess high density of ~0.86 g·ml−1 [12], which doesn’t change greatly with increasing temperatures [13]. Therefore, for example, when pyrolyzing plants in a wildfire emit α-pinene, its higher density relative to air allows it to migrate downwind at ground level and concentrate in nearby confined topographies such as canyons [14]. If accumulated, α-pinene will degrade under the thermal stresses of the approaching fire front [5]. Normally, the thermal degradation products of BVOCs (e.g. α-pinene), form a mixture of their VOC isomers in addition to aliphatic and aromatic hydrocarbons [15] [16]. When an accumulation of these gases is favoured by atmospheric conditions, it becomes possible for them to reach a lower flammability limit (LFL) [17], which is defined as the lowest concentration in air at which a combustible gas or vapor can ignite and propagate flame in the presence of an ignition source (spark, flame, heat), albeit such an ignition source will not be necessary if their LFLs were coupled with their auto-ignition temperatures (AIT). Notably, common BVOCs have an LFL of lower than 1% vol in air [18] and an auto ignition temperature (AIT) of 300˚C [19]. α-Pinene, for example, has an LFL of 0.81 and an AIT of 255˚C [20]. It remains important to identify the thermal degradation products of these BVOCs (e.g. α-Pinene) in order to also link their flammability characteristics (LFL, UFL & AIT) to the probability of a flashover in a wildfire.
Low temperature analytical pyrolysis studies on α-pinene are many and they primarily address its isomerization and rearrangement products however, the studies identifying the total range of its thermal degradation products at high temperatures are rather few [21] [22] [23]. This article covers the total range of the compounds emitted from the thermal degradation of α-pinene at temperatures similar to those reached in wildfires and describes the probable chemical reactions responsible for their production with the help of literature. In this work, α-pinene was thermally degraded in an inert atmosphere using a tubular oven set at a temperature range (300˚C - 900˚C).
2. State of the Art
2.1. BVOC Emissions and Volatility
2.1.1. Constitutive BVOC Emissions
Plants, particularly trees, emit considerable amounts of different constitutive biogenic volatile organic compounds (BVOCs) with a global average of 1150 Tg·C·year−1 [24]. Plant BVOCs are mainly terpenoids (hemi-, mono-, sesqui-, and diterpenes) [25], which are involved in plant growth, reproduction, defense, and communication within plant communities [26] [27] [28]. They are also strongly related to plant flammability [29]. The 5-carbons isomers isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP) are responsible for the biosynthesis of terpenoids [30]. On the basis of C5 units involved in their synthesis, terpenoids can be classified into hemiterpenoids or isoprene (C5H8), monoterpenes (C10H16), sesquiterpenes (C15H24), diterpenes (C20H32), and other polyterpenoids [31] [32]. The production precursors and the functional characteristics of the main BVOCs are reported in (Table 1).
2.1.2. Induced BVOC Emissions
When plants are exposed to various abiotic (extremes in temperatures, Ozone (O3), radiation, drought, etc.) or biotic (fungi, bacteria, herbivores, etc.) stresses, their constitutive BVOC emissions become induced (IBVOCs). Isoprene followed by monoterpenic hydrocarbons (C10H16) dominated by α-pinene, have been reported to be the most abundant BVOCs emitted from Mediterranean plant species during pyrolysis testing at moderate temperature ranges [33] [34]. However, analyses of smoke samples from wildfire plumes in Mediterranean forests, where temperatures are likely to exceed 900˚C, showed remarkable concentrations of BVOCs (isoprene, α-pinene, β-pinene) and aromatics (toluene and benzene), when compared to their concentrations in background air (Table 2).
2.1.3. BVOC Volatility
In the events of wildfires, IBVOCs originate from plants’ storage pools or de novo synthesis with proportions depending on the duration and severity of the thermal stresses, the plant species (with or without BVOC storage compartments), and the BVOC volatility [35]. The volatility of a BVOC is defined as the partition between its liquid and gas phase in the plant under high temperatures and it is measured by Henry’s law constant (Kh) [36]. Indeed, the compounds with a larger Kh partition primarily to the gas phase whereas those with a low Kh partition mainly to the aqueous phase [37]. The Kh of common BVOCs at 25˚C are listed in (Table 3). The Henry’s law constant of α-pinene at 25˚C is 13590 Pa·m3·mol−1 and is augmented by 1.3- to 1.8- fold for every 10˚C increase in temperature [38]. Therefore, our choice to study the thermal degradation of α-pinene is justified by its high volatility, coupled with its highest emission rates among other monoterpenes from Mediterranean plants during wildfires [34] [39].
![]()
Table 1. Main group of plant-produced biogenic volatile organic compounds (BVOCs) and their functional characteristics, [25].
![]()
Table 2. Concentrations of the main BVOCs and aromatics in background air and wildfire smoke plume, [8].
![]()
Table 3. Henry’s law constants (Kh) at 25˚C of common BVOCs.
aCopolovici and Niinemets, 2005 [38]. bCopolovici and Niinemets, 2015 [40].
2.2. Pyrolysis of α-Pinene in the Literature
According to the literature demonstrated in this section, the pyrolysis of α-pinene begins by its isomerization and rearrangement and isoprene formation at moderate temperatures ranging from 350˚C to 500˚C, followed by the formation of aliphatic hydrocarbons at elevated temperatures from 600˚C to 700˚C, and finally aromatic hydrocarbons at temperatures exceeding 700˚C.
2.2.1. α-Pinene Pyrolysis to Isoprene
The works of Hlasiwetz and Hinterberger, Schultz, Mokiewski, Tilden, and Mahood about the thermal degradation of terpenic hydrocarbons (mainly α-pinene) in the temperature range from 300˚C to 800˚C, were gathered in [23]. They found that α-pinene degrades in the temperature range from 300˚C to 450˚C to produce isoprene (C5H8) also known as (2-methyl-1,3-butadiene). Isoprene reaches a maximum production between 550˚C and 600˚C and degrades after that to produce a range of compounds. In the range from 400˚C to 500˚C, isoprene gets partially polymerized to higher unsaturated hydrocarbons of terpenic nature, while its transformation from 600˚C to 700˚C is almost complete to produce gases such as hydrogen (H2), saturated hydrocarbons such as methane (CH4) and unsaturated hydrocarbons such as ethylene (C2H4), propylene (C3H6), and 1,3-butadiene (C4H6). Aromatic compounds such as benzene (C6H6), toluene (C7H8), and xylene (C8H10) begin to appear at 700˚C and become the exclusive products above 800˚C. The percentages of the gases formed from the pyrolysis of isoprene in a range from 400˚C to 800˚C, are: 13.6% unsaturated hydrocarbons as ethylene, 58% - 66.3% methane (CH4), and 19.9% - 25.3% hydrogen, while the tar collected contained 10 grams of benzene, 53 grams of toluene, and 21 grams of naphthalene (C10H8), [23].
2.2.2. Thermal Isomerization of α-Pinene
The thermal isomerization of α-pinene has been studied in detail at temperatures ranging from 300˚C to 500˚C and lead to the conclusion that the products are dipentene (racemic limonene) and allo-ocimene through its precursor ocimene [41]. Later works provided precision to the temperature values responsible for the evolution of the isomerization products (e.g. dipentene and allo-ocimene) and their percentages [42] [43] (Figure 1). The degradation of α-pinene is negligible at 300˚C, then it undergoes a conversion of 25% and 75% at 360˚C and 395˚C, respectively [42]. As shown in (Figure 2), α-pinene conversion in the temperature range from 350˚C to 500˚C results primarily in 45% of limonene (4) and 2% of reactive ocimene which rapidly rearranges to alloocimene (3). Compounds (3) and (4) have maximum concentrations at 400˚C while other compounds such as pyronene (cp), is classified as a secondary reaction product from (2) and (4).
2.2.3. α-Pinene Pyrolysis to Aliphatic Hydrocarbons
The pyrolysis of isoprene produced from α-pinene above 400˚C, leads to hydrogen (H2), methane (CH4), and ethylene (C2H4). The latter will continue to degrade under the influence of temperature, [23]. Pyrolysis experiments conducted on orange bagasse from 350˚C to 600˚C showed considerable amounts of α-pinene in the collected pyrolysis oil, while the pyrolysis gas content was dominated by methane (CH4) with a remarkable increase in its production starting from 400˚C,
![]() (a)
(a) ![]() (b)
(b)
Figure 1. Schemes of the thermal isomerization of α-pinene into dipentene (limonene) and allo-ocimene through ocimene. (a) Stolle and Ondruschka [42]; (b) Anikeev [43].
![]()
Figure 2. Yield of pyrolysis products of α-pinene (1), depending on reaction temperature (T). (2) ocimene; (3) alloocimene; (4) limonene, [42].
[44]. The coupled effect of methane abundance and increasing pyrolysis temperature also increased the yield of lower hydrocarbons such as acetylene (C2H2), ethane (C2H6), and propane (C3H8), [44]. Methane may not have been formed from α-pinene solely, but it is certain that the increase in the yield of ethyne, ethane and propane were mainly dependent on the increasing methane concentration in the pyrolysis process [44]. Therefore, it is reasonable for us to analyse the pyrolysis products of methane from α-pinene as it appears to be the main source of aliphatic and aromatic compounds. The pyrolysis of methane is a very complex process because it involves several radical reactions that have been studied extensively in the literature [45] [46] [47]. The following will provide a detailed step by step degradation analysis of methane to flammable hydrocarbons and later to aromatics through 1,3-butadiene in the temperature range from 700˚C to 900˚C as suggested in [48], (Figure 3).
A large number of reactions occur when methane is heated. However, the predominance of specific reactions is a function of temperature and composition of the gas mixture. The first reaction from methane pyrolysis at 700˚C is the dissociation reaction in (Figure 3(a), (stage 1)) where a methane molecule splits to form a methyl radical and a hydrogen radical in reaction (1) followed by the formation of ethane and hydrogen in reaction (2).
(1)
(2)
As more ethane is formed it undergoes molecular scission in a highly endothermic reaction to produce methyl radicals in reaction(3). While reaction (2) continues, ethane undergoes an indirect dehydrogenation within seconds under the influence of heat to produce ethylene (C2H4) in (Figure 3(a), (stage 2)) through
![]() (a)
(a) ![]() (b)
(b)
Figure 3. Yields from the pyrolysis of methane (CH4), [48]. (a) 750˚C; (b) 850˚C.
series of reactions (4) to (7)resulting in decreased rate of ethane formation and increased ethylene production.
(3)
(4)
where,
(5)
(6)
(7)
The yield of ethylene reaches a maximum at 726˚C and gradually decreases beyond this temperature simultaneously with the decrease in methane as no more methyl radicals can be generated. In (Figure 3(a), (stage 3)), ethylene degradation produces acetylene (C2H2) in reaction (8) and propene (C3H6) in reaction (9). The kinetics of these reactions and their activation energies can be obtained from [48].
(8)
(9)
2.2.4. α-Pinene Pyrolysis to Aromatic Hydrocarbons through the Unimolecular Decomposition of 1,3-Butadiene
The yield of benzene (C6H6) from the pyrolysis of pine chips which are rich in α-pinene ranged from 0.071% of emissions at 500˚C to a maximum of 3.13% at 900˚C [49]. An important role in aromatics formation from α-pinene is played by 1,3-butadiene (C4H6) which is formed in the pyrolytic processes of isoprene and methane from 600˚C to 700˚C [23] [48]. However, the production of aromatics from 1,3-butadiene is suggested to occur through radical mechanisms instead of the Diels-Alder reaction which is not favoured above 400˚C, [48]. The most important reactions in the formation of benzene from methane pyrolysis through 1,3-butadiene are illustrated in (Figure 4). Literature provided several reaction pathways for the formation of aromatics from 1,3-butadiene after its unimolecular decomposition to vinyl radicals (C2H3·) at 700˚C through reaction (10).
(10)
A vinyl radical (C2H3·) reacts with acetylene (C2H2) to produce butadienyl-radical C4H5·, which in turn reacts with acetylene (C2H2) to produce benzene (C6H6) and release a hydrogen radical as in reactions (11) & (12), [50].
(11)
(12)
Above 700˚C one molecule of vinyl radical reacts with one molecule of 1,3-butadiene to produce a 1,3-cyclohexadienyl radical. The later loses a hydrogen radical to produce 1,3-cyclohexadiene which leads to benzene and hydrogen in reaction (13), [48].
![]()
Figure 4. Reaction network for the pyrolysis of methane, and production of benzene (C6H6) from unimolecular decomposition of 1,3-butadiene (C4H6), [50].
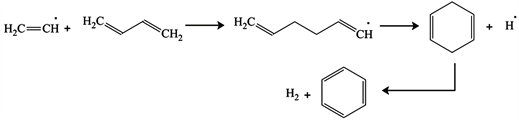 (13)
(13)
2.2.5. α-Pinene Pyrolysis to Aromatic Hydrocarbons through the Dimerization of 1,3-Butadiene
Aromatics can also be formed from 550˚C to 700˚C after the dimerization of 1,3-butadiene into 4-vinylcycloxenes (C8H12). Literature addressed this mechanism to refute the aromatization scheme based on Diels-Alder reaction between butadiene and a lower olefin. Aromatization schemes of 1,3-butadiene at 550˚C and 700˚C, showed that the presence of an olefin is not necessary to produce the precursors of C6 to C8 aromatics through the Diels-Alder reaction, especially that this last is not favored above 400˚C [48] [51]. It is also suggested that cyclohexene (C6H10) formed from the reaction of 1,3-butadiene with an olefin (i.e. ethylene or propene) in Diels-Alder reaction, can’t undergo direct dehydrogenation to produce benzene (C6H6). Even in the presence of a cracking catalyst, the cyclohexene formed will only yield to 7% of benzene and 4% of toluene at 550˚C, [51]. On the other hand, the dehydrogenation of a cyclohexadiene (C6H8) to produce benzene (C6H6) should be easier because both of the hydrogens to be removed are allylic [51]. The alternative schemes of 1,3-butadiene aromatization begin by its dimerization to 4-vinylcyclohexenes (C8H12) from 550˚C. 4-vinylcyclohexenes (C8H12) undergo double bond and skeleton isomerization to produce C8 aromatics (styrene), and splitting of one and two of their carbon atoms to form C7 aromatic (toluene) in reaction (14) and C6 aromatic (benzene) in reaction (15), [51]. At 550˚C, styrene production (2.4 moles/100 moles of butadiene) dominates the production of toluene (1.10 moles) and benzene (0.9 moles) while at 700˚C benzene and toluene predominate at 11.71 and 7.22 molar % of butadiene, respectively [51]. Toluene (C7H8) production from 4-vinylcycloxene is accompanied by an equimolar amount of methane (CH4) in reaction (14), while the production of benzene produces an equimolar amount of C2 hydrocarbons in reaction (15).
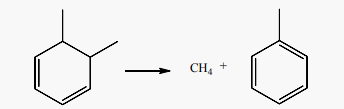
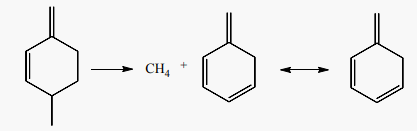 (14)
(14)
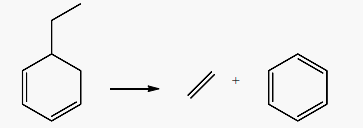
 (15)
(15)
Benzene can also be produced from toluene via the highly selective demethylation reaction which occurs when toluene is heated from 626˚C to 726˚C in the presence of excess hydrogen to produce benzene and methane in reaction (16), [52].
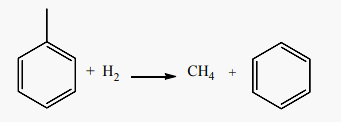 (16)
(16)
In this section we demonstrated the information collected from literature about the stages of α-pinene pyrolysis to isoprene, then to its monoterpenic-isomers, aliphatic hydrocarbons (methane, ethane) and finally to aromatics through 1,3-butadiene. The following sections demonstrate the formation of these products through our pyrolysis experiments on α-pinene in an inert atmosphere (N2) using a tubular furnace set at a temperature range from 300˚C to 900˚C.
3. Materials and Methods
6 ml liquid sample of α-pinene (purity > 98%) from Sigma Aldrich®, was placed in a crucible with a rod (nacelle) equipped with a thermocouple (0.16 mm silica coated chromel – alumel) to measure the temperatures developed at the sample level when introduced into the reactor in (Figure 5(a)). The nacelle was introduced in a tubular quartz reactor (80 cm long × 5 cm in diameter) connected to two gas chromatographs (GC) through heated transfer lines in order to avoid condensation of the pyrolysed products. The reactor was swept for 5 min with nitrogen to remove oxygen from the system. The quartz tube was then placed in a 40 cm long × 6.5 cm diameter tubular furnace heated at different temperatures ranging from 300˚C to 900˚C with a step of 50˚C. At the desired temperature, the sample was heated for 2 mins then the pyrolysis products were flushed by a nitrogen flow (1.5 L·min−1) to the GC for analysis.
Nitrogen allows the transfer of the vaporized sample from the reactor to the gas chromatographs (GC). Two gas chromatographs (GC) were used for the vaporized sample analysis, a PeriChrom 2100 (A) and a Varian CP 3800 (B) (Figure 5(b)). Each chromatograph is equipped with columns and two detectors; a flame ionization detector (FID) and a thermal conductivity detector (TCD) (Table 4).
Hydrogen can be detected with TCD when the reference gas is helium, but the response is very low because the thermal conductivity of hydrogen (0.1805 W·m−1·K−1) and helium (0.1513 W·m−1·K−1) are very close. On the contrary, the difference between the thermal conductivities of argon (0.017 W·m−1·K−1) and
![]() (a)
(a) ![]() (b)
(b)
Figure 5. Experiment apparatus. (a). The tubular furnace with α-pinene nacelle and connection lines assembly; (b). Perichrom (A) and Varian (B) chromatographs.
![]()
Table 4. Detectors of chromatographs (A) & (B) their columns, and the detected compounds.
hydrogen is high which makes argon more interesting for the hydrogen detection using TCD. For this reason, we relied on the hydrogen readings of the TCD of (B) which uses argon as a reference gas, contrarily to (A) which uses helium.
Notably, the vapor colour changed from white into beige at 700˚C and brown at 800˚C and 900˚C. Once the vapor was visible at the chromatograph entrance, the analysis was carried out after a delay of 30 seconds to allow the mixture to fully enter the columns. Between the different trials, the oven was shut down and the apparatus was purged with nitrogen flow for 30 mins. The uncertainity of our results depended on several factors including the stability of the experimental apparatus, precision of data acquistion, and the atmospheric conditions (pressure, temperature). However, based on the relative standard deviation in the percentages of the compounds identified from the experiments repeated twice at the same temperatures, the reproducibility of our results was found to be 75%.
4. Results and Discussion
At 300˚C no degradation products of α-pinene were observed. This result is consistent with the results of the oxidative degradation of α-pinene from 300 to 900˚C in [22]. Therefore, the following analyses will be carried out on the products formed from 400˚C to 900˚C. The relative concentrations of the compounds detected by each detector were normalized according to the total sum normalization (TSN) technique and plotted according to the separation range of each column.
4.1. Monoterpenes and Isoprene
Through the FID detector of (B), we detected monoterpenes (C10H16) and isoprene (C5H8), and compared them to the findings in [41] (cf. section 2.2.1 & 2.2.2). At 400˚C, the vapours were composed of 19% α-pinene and 65% monoterpenes as shown in (Figure 6). Both followed a decreasing profile beyond 400˚C. However, the notable concentration of monoterpenes from 500˚C, even after the complete consumption of α-pinene, could be explained by the presence of limonene and pyronene. Isoprene (C5H8) production from α-pinene in an inert gas atmosphere begins at 500˚C while the maximum production is obtained at 600˚C consistent with the suggestions in [23] and [53].
![]()
Figure 6. Normalized concentrations of monoterpenes and isoprene produced from α-pinene degradation measured by the FID of Varian (B).
4.2. Aliphatic Hydrocarbons and Hydrogen
During α-pinene pyrolysis experiments, the TCD of (B) detected methane (CH4) and hydrogen (H2), (Figure 7(a)). Methane and hydrogen production schemes follow that of isoprene from 400˚C to 600˚C, then their concentrations boost in parallel with isoprene degradation starting from 600˚C (cf. Section 2.2.1). The noticeable increase in hydrogen concentration beyond 700˚C can be explained by methane degradation (cf. Section 2.2.3) butadiene aromatization (cf. Section 2.2.4 & 2.2.5). The FID of (A) measured concentrations of ethane, ethene, propene, and propane (Figure 7(b)) which allowed us to correlate their production schemes to the pyrolysis processes of isoprene and methane.
The production of propane (C3H8) and ethane (C2H6) from 400˚C to 550˚C in (Figure 7(b)) can be referred to the coupled effect of methane abundance and high temperatures [44] (cf. Section 2.2.3). Ethane and hydrogen formation following that of methane from 400˚C is consistent with (Figure 3(a), (stage 1)), and reaction (2) where two methane molecules combine to form ethane and hydrogen. With no apparent isoprene concentrations from 600˚C as shown in (Figure 6), the rate of ethane formation decreases and ethene production boosts as in (Figure 3(a), (stage 2)), and reaction (4) [23] [50]. Propene (C3H6) concentrations in (Figure 7(b)) can be referred to the pyrolysis of isoprene from α-pinene between 400˚C and 800˚C as suggested in [23]. Also, propene and hydrogen were found in propane pyrolysates from 650˚C to 800˚C [46], which can explain why propene concentrations remain larger than propane in this temperature range, (Figure 7(b)). The overall reactions of the compounds formed from methane can be described by the stepwise dehydrogenation reaction (17) [48] [50].
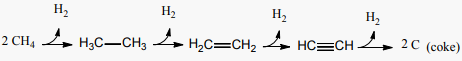 (17)
(17)
Acetylene (C2H2) was not detected by the Perichrom FID during our experiments despite that its formation was suggested to result from the degradation of
![]() (a)
(a)![]() (b)
(b)
Figure 7. Normalized concentrations of compounds produced from α-pinene degradation at different temperatures measured by: (a) TCD of Varian (B). (b) FID of Perichrom (A).
butadiene below 400˚C or from the various interactions of free radicals and molecules during the pyrolysis process of ethane beyond 764˚C [48]. Acetylene is necessary for benzene formation via reactions (11) & (12) [50], however, our results do not support this step because unless acetylene was directly degraded upon formation, we couldn’t trace it in our experiments.
4.3. 1,3-Butadiene and Aromatics
The production scheme of 1,3-butadiene (C4H6) follows that of isoprene (Figure 8). Significant concentrations of aromatics such as benzene and toluene were measured from 550˚C by the FID of the Varian (B) (Figure 8).
The literature in sections 2.2.4 & 2.2.5 presented a refutation to the formation of aromatics through Diels-Alder reaction between 1,3-butadiene and an olefin. Instead, the unsaturated structure of 1,3-butadiene makes it play an important role in the formation of poly-aromatic hydrocarbons (PAH) through its own
![]()
Figure 8. Normalized concentrations of benzene, toluene, and 1,3-butadiene (C4H6) produced from α-pinene degradation at different temperatures measured by the FID of the Varian (B).
pyrolysis [54]. As suggested in Section 2.2.5 with reference to [51], the dimerization of 1,3-butadiene results in the production of benzene and toluene from 550˚C with humble concentrations as shown in (Figure 8). At 700˚C the decrease in 1,3-butadiene indicates the initiation of its degradation assumingly to vinyl radicals (C2H3·) through reaction (10) and is accompanied by the increase in the formation of benzene and toluene from 700˚C probably through reactions (13), (14), and (15). However, the constant concentration of hydrogen from 550˚C to 600˚C and from 700˚C to 750˚C may be an indication that no direct dehydrogenation occurs during the production of aromatics which can support the refutation of the aromatization scheme through the Diels-Alder reaction. On a separate note, the fluctuating concentration of toluene along with a continuous increase in benzene can be referred to reaction (16) signalling the production of benzene from toluene from 600˚C to 700˚C and from 750˚C to 900˚C, [52].
5. Conclusion
Gases formed from the pyrolysis of α-pinene in a tubular furnace at a temperature range from 300˚C to 900˚C were monitored by chromatography. α-Pinene thermal decomposition is a complex and temperature dependent process. The temperature range adopted in our experiments resulted in a mixture containing aliphatic hydrocarbons (monoterpenes, paraffins, and olefins) and aromatics. Isoprene production was observed to reach a maximum between 550˚C and 600˚C. Methane production from α-pinene was observed to dominate before its degradation at 700˚C. Abundance of methane and the increased pyrolysis temperature was coupled with an increase in the yield of lower hydrocarbons as propane and ethane. This later degrades to yield ethene. Benzene and toluene were the main aromatics produced while their formation was referred to reactions of radical mechanisms instead of the conventional Diels-Alder scheme. The evolution of the pyrolysis products of α-pinene in a wildfire will certainly participate in flame propagation. However, their accumulations ahead of the fire front in topographic relief depressions such as canyons, coupled with their flammability characteristics (LFL, AIT) are being currently investigated in relation to wildfire flashover phenomena. While α-pinene is considered the most abundant monoterpene emitted from the plants of the Mediterranean climate, it remains important to undergo pyrolysis testing on the complete range of the BVOCs emitted during wildfires in order to configure the behaviour and gaseous identities of their product yield under significant fire temperatures.