Physicochemical and Analytical Studies of Some Monomer and Polymer Complexes Derived from Selected Aroyl Hydrazone ()
1. Introduction
It is well known that hydrazones occupied a central role in the development of coordination chemistry. This feature comes from the fact that the hydrazones, derived from the condensation of o-hydroxyl or methoxy aldehydes and ketones with hydrazides, are potential polynucleating ligands possessing azomethine and phenol or methoxy functions [1] offering varying bonding possibilities in metal complexes. Studies of some metal chelates of hydrazones derivatives are well known in literature [2] - [10].
On introducing the SO2 group instead of the carbonyl group (CO) of the hydrazones moiety, the compounds are called sulphonyl hydrazones having the following formula, RSO2NHN=CHR’. Bivalent metal complexes of benzene sulphonyl hydrazide (BS) have been studied [11]. It was reported earlier that BS coordinates to the central metal ion through the SO2 and nitrogen of the NH group with the removal of NH proton where a polymeric chain has been suggested [12]. Moreover, salicylidene benzene sulphonyl hydrazone (HSBS) with some transition metals (M = Co2+, Ni2+, Cu2+, Hg2+, Zn2+, and Cd2+) was synthesized and characterized by different physicochemical techniques. The results showed that Ni2+, Cu2+, and Hg2+ form complexes with the general formula, [M(SBS)2], while Co2+, Zn2+, and Cd2+ form complexes with the polymeric formula [M(SBS)2]n. The ligand acted in the latter case in a bidentate fashionvia the NH and SO2 groups (with deprotonation of the NH group forming a polymeric structure [12].
Finally, Cu2+ and Ni2+ complexes of hydrazones derived from benzene sulphonyl hydrazine with salicylaldehyde and 2-hydroxy-1-naphthaldehyde were studied by physical and spectral methods [13]. Based on elemental analyses and spectral (i.r. and n.m.r.) data the results suggest that the isolated hydrazones behave as monobasic bidentate towards the metal cations and coordinate through the C=N and deprotonated phenol OH groups. It is worth mentioning ion that the spectral (Uv-vis.), magnetism as well as thermal (TG, DTG, and DTA) measurements were not been investigated for these complexes. In addition, some data obtained in this work differs from that reported before [13].
The goal of this paper is to synthesize and characterize the stereochemistry of the solid complexes derived from the reaction of 2-hydroxy-1-naphthaldehyde-benzene-sulphonylhydrazone (HNB), p-toluene-(HNT) with Co2+, Ni2+ and, Cu2+ salts. The effect of the methyl group that represents the only difference between the two synthesized hydrazones on their coordinating nature has been illustrated. Furthermore, the pKa values of HNB and HNT were determined and the stoichiometry of complexes in solution has been determined by spectrophotometric and conductance techniques.
2. Experimental
All chemicals and solvents utilized were of BDH or AR quality and used without further purification. 1H-NMR spectra in d6-DMSO with TMS as internal standard were obtained from a Jeol-FX-90Q Fourier NMR spectrometer at Cairo University, Egypt. IR spectra were recorded as KBr pellet in the range “200-4000 cm−1” on a Mattson 5000 FTIR spectrometer at Mansoura University. Mass spectra of the ligands were performed by a Shimadzu-GC-MS-QP1000 EX using the direct inlet system at Cairo University, Egypt. Elemental analyses were determined using Perkin-Elmer 2400 at Cairo University, Egypt (Table 1). Metal contents were estimated using EDTA as reported earlier [14] [15] [16]. UV-vis. spectra of the samples in Nujol mull or DMSO, and in DMSO were measured in the range of 200 - 1000 nm using a Unicam UV2-100 spectrophotometer at Mansoura University, Egypt. The mass susceptibility of the solid complexes (cg) was measured with a magnetic susceptibility balance of model Sherwood at Mansoura University, Egypt. The thermal analyses (TG, DTG, and DTA) under nitrogen were carried out with a Shimadzu 50 H thermal analyzer at Mansoura University, Egypt.
2.1. Preparation of Benzene Sulphonyl Hydrazine (BH)
Benzene sulphonyl hydrazine (BH) was prepared as described earlier in the literature [11]. The isolated product was crystallized from bi-distilled water and dried in the air. A yellowish-white crystal was obtained (Mp. = 94˚C).
2.2. Preparation of Hydrazones Ligands (HNB, HNT)
The two ligands, HNB and HNT, were synthesized by adding BH (0.02 mol, 3.44 g) dissolved in absolute methanol (50 mL) to a solution (50 mL) of 2-hydroxy-1-naphthaldehyde (0.02 mol, 3.44 g) (HNB) and 2-hydroxy-1-naphthaldehyde p-toluene sulphonyl hydrazone (HNT), respectively. The reaction mixtures were refluxed for 3 h in a water bath (95˚C). The product is separated out by concentrating the solution to half of its volume and cooling. The crystals of the desired ligand were filtered off and finally recrystallized from methanol. The chemical structures of the resulting hydrazones are shown in Figure 1.
2.3. Preparation of Solid Complexes
All the isolated solid complexes (Table 1) were prepared by mixing equivocal amounts of ligands and M(II) acetates (M = Co2+, Ni2+, and Cu2+) in 100 mL ethanol. The reaction mixture was refluxed in a water bath (95˚C) for 6 h. The colored microcrystalline solids were isolated by filtration on hot, washed repeatedly
![]()
Figure 1. Chemical structures of hydrazones.
with hot ethanol, and ether, and finally dried in an oven at 80˚C for 24 h.
2.4. Preparation of Solutions for Spectrophotometric Measurements
The appropriate concentration of the metal ions (Co2+ and Fe3+) and ligands were mixed in an absolute methanol solution. The final volume of the mixture was always kept constant by adding absolute methanol. All the absorbance measurements were recorded in the range “200 - 800 nm”. Methanol was used as a blank in the case of Fe3+ complexes while the ligand was used as a blank in the case of Co2+ complexes.
3. Results and Discussion
All the analytical, physical, and spectroscopic data of the hydrazones and their isolated metal complexes are recorded in Table 1 & Table 2. A comparison of the analyses for both the calculated and found percentages indicates that the composition of the isolated solid complexes coincides with the proposed formulae. All trails to isolate mono- or tris-ligand chelates by direct reaction of the ligands with the M(II) salts were unsuccessful. The complexes are air-stable for a long time, insoluble in water, alcohols, and carbon tetrachloride, and soluble in DMF and DMSO.
![]()
Table 1. Analytical, physical and spectroscopic data of the hydrazones and their complexes.
![]()
Table 2. IR and electronic absorption data of hydrazones and their complexes.
disappeared on adding D2O, b(Co-HNT system, λmax = 385 nm), c(Fe-HNT system, λmax = 530 nm), d(Fe-HNT system, λmax = 670 nm.
3.1. IR Spectra
The positions of the IR bands of hydrazones and their metal complexes are summarized in Table 2. The IR spectra of HNB and HNT showed a strong band at 1620 - 1624 cm−1 assigned to υ(C=N) of the azomethine. The observation of this band emphasizes the formation of the azomethine linkage. Each ligand also has a strong band in the region of 700 - 800 cm−1 corresponding to the out-of-plane deformation of the aromatic rings. The observation of broad but weak bands in the 2000 - 1700 cm−1 region for HNB and HNT is taken as evidence for the formation of a stable six-membered ring of intermolecular hydrogen bond of the type OH…N [17] .
The IR spectral data for Co2+, Ni2+ and Cu2+-HNB complexes pointed out that HNB behaves as a bidentate ligand coordinating to one metal ion via the SO2 and OH groups with a displacement of a proton from the latter group tending to form a polymeric chain structure (Figure 2). A worthy mention, it was reported that the Co2+ ion forms a polymeric chain with salicylidene benzene sulphonyhydrazone [12]. In fact, this suggestion is assumed on the basis of the following pieces of evidence (Table 2): 1) The characteristic main band of the SO2 group at 1318 cm−1 assigned to νas(SO2) shifts to a higher wavenumber by 10 - 14 cm−1 indicating that this group is taking part in bonding, 2) the disappearance of both ν(OH) and δ(OH) bands elucidates the deprotonation of this group, 3) the positive shift of ν(C-O) band indicates the formation of (–C-O-M) bond. Nevertheless, C=N was expected to involve in coordination in the case of HNB complexes, it was excluded here due to the absence of any significant shift or variation in its position or intensity upon chelating. This result is not consistent with the previously published data [12] [13]. Meanwhile, HNT coordinates in a bidentate fashion to one metal ion via the azomethine nitrogen (C=N) and OH groups (Figure 3). Displacement of a hydrogen proton from the latter group leads to a
![]()
Figure 2. Proposed structures of the HNB complexes.
![]()
Figure 3. Proposed structures of the HNT complexes.
six-membered ring around the central metal ion. This behavior is proposed on the basis of (Table 2): 1) the characteristic three main bands of the SO2 group at 1318, 1167 and 574 cm−1 remain more or less at the same positions excluding the participation of this group in coordination, 2) the bands of both ν(OH) and δ(OH) vibrations disappear, and the ν(C-O) band is shifting to a higher frequency, 3) the negative shift of the azomethine group to lower wavenumber confirming the involvement of this group in bonding [18]. In spite of this group is not taking part in coordination in the case of HNB which is nearly resembled in its chemical structure to HNT, it is involved here. This can be interpreted on the basis of the presence of an electron-donating group (methyl) in the pare position for -SO2NHN=C. This group increases the electron density on the azomethine nitrogen (N=C) of HNT (in comparison to HNB) and subsequently, its donating nature increases facilitating its linking to the metal.
Indeed, the appearance of ν(NH) band with its shift to higher wavenumber in all synthesized complexes may be due to the destruction of the intermolecular hydrogen bonding between the OH and C=N groups or presumably due to the rupture of an intermolecular hydrogen bond of the type N-H…..N-H suggesting of this intermolecular H-bonding arises from the observation of δ(NH) in the 1H-NMR spectra of HNB and HNT at a higher position compared with that reported in the literature. Moreover, the persistence of new bands in the IR spectra of metal complexes as shown in Table 2 assigned to M-O and/or M-N indicates the formation of the metal complexes. The observation of only one band for each Co-N and Co-O group suggests the existence of trans-configuration [19]. Strong broad absorption in the 3300 - 3600 cm−1 region for metal complexes substantiates the presence of water and/or ethanol.
3.2. 1H-NMR Spectra
The assignments of the main signals in 1H-NMR spectra of the ligands under investigation are shown in Table 1. 1H-NMR spectra of hydrazones exhibit three signals with equal integration (1:1:1) at (11.59, 11.2, 8.85) and (9.8, 9.4, 7.0) ppm, downfield of TMS, and assigned to the protons of (NH, OH, CH=N) of HNB and HNT, respectively. On adding two drops of D2O, the NH and OH signals disappeared while the signal of CH=N remained. The higher frequency of the OH signal(s) compared with that reported in the literature is most probably due to the intermolecular hydrogen bonding operating between the hydrogen atom of the ortho-hydroxyl group and the azomethine nitrogen (OH…N-type) as previously confirmed by the IR data. By comparing the 1H-NMR spectra of HNB (HL1) with its relating Ni(II) complex namely, [Ni(L1)2(EtOH)1/2]∙2H2O, it is evident that 1) no signal is recorded for a phenol hydroxyl proton at 11.2 ppm, as in case of the HNB hydrazones indicating deprotonation of the ortho-hydroxy group 2) the remaining of the signals attributed to the proton of NH and CH=N groups at nearly the same positions (Table 1) confirms the un participation of these groups in coordination and thus coincides with the obtained IR data and the proposed polymeric chain structure [20]. Perhaps, this comparison explains the coordination behavior of HNB with Co2+ and Cu2+ salts which are analogous complexes. It is well known that Co2+, Ni2+, and Cu2+ ions are resembled in their chemical behavior due to the convergence in their ionic radii.
3.3. Electronic and Magnetic Spectra
The electronic spectra of the hydrazones and their solid metal complexes [21] [22] [23] [24] as well as the geometry and magnetic data of the formed chelates are shown in Table 2. The redshift of HNT bands in comparison to that observed in the case of HNB (Table 2) is mainly due to the existence of the methyl group in the para-position. This electron-donating group increases the electron density on the O=S=O, C=N, and OH groups, and consequently, a redshift was observed. The electronic spectra of the metal chelate suggest different environments around the metal ions. The results revealed that Cu2+ complexes are pseudo-tetrahedral; NiII complexes are trigonal-bipyramidal while Co2+ complexes have square-planar geometry. For the magnetic studies, the values of μeff of all complexes may be considered in good agreement with the proposed structures [25] [26] [27].
3.4. Thermal Analysis
The thermal decomposition studies (TG, DTG, and/or DTA) on major solid metal complexes have been carried out. The TG curves up to 800˚C for complexes showed 3-5 stages of decompositions with the formation of metal carbonates/oxides/carbide or mixtures of them at the last stage. The results of the thermal analysis revealed that: 1) [Co(L1)2]n showed a first weight-loss stage at 219˚C assignable to the decomposition of the complex and at the same time emphasized the absence of any crystalline solvent. This temperature (219˚C) is high enough to be considered for out sphere ethanol or water. The TG curve is ended by the existence of CoCO3, 2) [Co(L2)2].1/2EtOH.1/2H2O decomposed at 275˚C and ended by CoCO3 as well. The two weak bands in the range 100˚C - 200˚C suggest the removal of the solvent (1/2EtOH + 1/2H2O), 3) [Ni(L1)2(EtOH)1/2]n∙2nH2O and [Ni(L2)2(EtOH)1/2] decomposed at 134 and 322˚C, respectively. The residue corresponds to the formation of nickel carbonate, oxide, and carbide. The first weight-loss stage at 74˚C in case of [Ni(L1)2(EtOH)1/2]n∙2nH2O corresponds to the removal of 1/2EtOH + 2H2O while the DTA diagram for [Ni(L2)2(EtOH)1/2] showed an endothermic peak at 90˚C corresponding to the removal of 1/2EtOH molecule from the complex, 4) TG curve of [Cu(L2)2].1/2EtOH showed a first decomposition stage at 216˚C. This stage is accompanied by an exothermic peak in the DTA curve indicating the starting of decomposition. On the other hand, the DTA showed an endothermic peak at 102˚C assignable to the removal of 1/2EtOH.
In the light of the foregoing results, the most reasonable structures of the M(II) chelates can be represented by Figure 2 and Figure 3, respectively.
The behavior of Co2+ and Fe3+ ions with HNT has been studied and the stoichiometry was determined. The other ligand under investigation (HNB) is not investigated since it precipitates a solid complex with Co2+ and gives unspecified color with Ni2+ and Cu2+ ions (i.e., a mixed color of HNB and metal (II) ion without any reaction).
3.5. Spectral Studies in Solution
Comparing the spectra of the Co2+, and Fe3+-HNT complexes with HNT and the employed metal salt solutions (cobalt acetate and ferric chloride) at room temperature showed a new band at 385 nm with a shoulder at 400 nm for Co2+-HNT and a band at 670 nm in case of Fe3+-HNT. Increasing the concentration of the ligand (from 1 ´ 10−4 to 1 ´ 10−4 M for Co2+-HNT and to 6 ´ 10−4 M for Fe3+-HNT) leads to a hyperchromic shift without changing the position of the band manifesting the formation and stabilization of the complex in solution. On leaving Fe2+-HNT solution for 42 h, it was noticed that the color of the solution has been changed from green to brown accompanied by a hypsochromic shift for the absorption maxima from λmax at 670 nm (green solution) to 530 nm (brown solution). This indicates that the Fe3+ complex formed at once is quite stable but attains maximum stability after 42 h. Besides, it suggests the increase of 10 Dq, and consequently, the geometry of the complex may be changed. From this view, the effect of temperature on a Fe3+-HNT is of considerable interest to be studied. Increasing the temperature from 25 to 55˚C for the Fe3+-HNT system reveals a hypsochromic shift for λmax from 670 to 560 nm (Figure 4). Doubtless, this indicates that the increase of temperature helps too much for reaching the stability of the formed complex.
To trace the complex formation and deduce the stoichiometry of the complexes in solution, continuous variation [28], the molar ratio [29], and slope ratio [30] methods were employed. For the Co2+-HNT mixture, the results obtained (at λmax = 385 nm) by continuous variation (Figure 5), molar ratio, and slope ratio indicated the formation of the complex with 1:1 (Co2+: HNT). On the
![]()
Figure 4. Effect of temperature on FeCl3-HNT complex.
other hand, the results of continuous variation, molar ratio, and slope ratio methods for Fe3+-HNT mixture at the λmax’s = 670 and 530 nm at once and after 42 h are in good agreement with each other and manifested the existence of 1:2 (Fe3+:HNT) and 2:1 (Fe3+:HNT) complexes, respectively. The continuous variation diagram (Figure 6, and Figure 7) revealed also a 1:1 (Fe3+:HNT) species at 530 nm, and high intensity for 2:1 (Fe3+:HNT) compared with 1:1 and 1:2 (Fe3+:HNT) indicating the higher stability of the 2:1 M:L complex. Based on the obtained results, it is suggested that 1:2 (Fe3+:HNT) was reached to 2:1 (Fe3+:HNT) passing by 1:1 (Fe3+:HNT). Also, It is very conspicuous that the stability of the 2:1 (Fe3+:HNT) complex increases with time and temperature. A literature survey pointed out that the octahedral
showed a band at 704 nm while the tetrahedral
showed a band at 588 nm [31]. According to this fact, the hypsochromic shift of λmax from 670 to 530 nm may be due to two probabilities (1) the geometry of the Fe3+-HNT complex was changed from octahedral to tetrahedral due to the steric effect of ligand or (2). The kind of ligands was varied
![]()
Figure 5. Continuous variation method for Co(II)-HNT complex.
![]()
Figure 6. Continuous variation method for Co(II)-HNT complex.
![]()
Figure 7. Continuous variation method after 42h for FeCl3-HNT complex.
but with preservation of the geometry i.e., the electronic field strength of ligands increased.
Spectrophotometric studies on the complexation of Co2+ and Fe3+ with HNT were performed in order to show the possibility of applying the reaction for the micro-determination of those two metal ions. Both Co2+ and Fe3+ ions are only selected within the all investigated meal ions since they exhibit a change in color which can be detected in the visible region. For this purpose, absorbance measurements were carried out by adding the metal salt solution in varying concentrations to a constant concentration of HNT in absolute MeOH. On plotting the obtained absorbance values at the above selected wavelengths (385, 670, and 530 nm) against the corresponding concentration of the metal ions to verify Beer’s law, linear relations were obtained (calibration curve). At a higher concentration of Mn+, a negative deviation was observed. The results suggested that Beer’s law is obeyed for 1) Co2+-HNT system at λmax = 385 nm up to 0.1 ´ 10−3 M, 2) Fe3+-HNT (at once and RT) at, λmax’s=530 and 670 nm up to 0.5 ´ 10−3 M and 3) Fe3+-HNT (after 42 h) at, λmax’s = 530 and 670 nm up to 2 ´ 10−3 M. The sensitivity y values (Table 1) were estimated by Sandall’s method [32] following the equations:
where:
y = sensitivity (number of micrograms of an element present as the absorbing species in a column solution of 1 cm2 cross-section giving an absorbency of 0.001 at specified wavelength).
n = number of elements in a molecule of the compound.
At.wt = atomic weight of the element in gram.
e = molar absorptivity.
A = maximum absorbance obeyed Beer’s law.
c = concentration corresponding to the absorbance A.
b = thickness of the utilized quartz cell (1 cm).
3.6. Effete of pH on the Absorption Spectra of HNT and HNB
Absorption spectra of HNT (1 ´ 10−4 M) and HNB (1.2 ´ 10−5 M) in universal buffer solutions were recorded in the range of 200 - 450 nm. The spectra exhibit one band in the pH range 8 - 12 at 353 and 243 nm for HNB and HNT, respectively. No absorbance was traced in the lower pHs (1 - 7) in the case of HNB owing to the participation that occurred. The relation between absorbance versus the pH was represented in Figure 8 and Figure 9 shows the S-shape curve. The pKa values were calculated from these curves and located in Table 1. The
![]()
Figure 8. Effect of universal buffer on HNB ligand.
![]()
Figure 9. Effect of universal buffer on HNT ligand.
smaller pKa value of HNB (6.5) compared with that of HNT (9.5) arises from the effect of the electron-donating nature of the (CH3) group in HNT which increases the electron density on the OH (naphthyl) group and consequently, the pKa was observed at high pH value. On the other hand, the existence of a withdrawing group (phenyl) in HNB decreases the electron density on the OH (naphthyl) group and hence the pKa has a small value.
Utilizing absolute methanol solutions, conductance titration of 10 mL (2.5 ´ 10−4 M) cobalt acetate solution with HNB (2.5 ´ 10−3 M) and 25 mL of cobalt acetate solution (5 ´ 10−4 M) with HNT (5 ´ 10−3 M) were carried out and represented in Figure 10 and Figure 11, respectively. The results of the Co2+-HNB curve (Figure 10) showed two breaks at 1:1 and 1:2 (Co2+:HNB). This illustrates that the two complex species with different ratios are possibly formed
![]()
Figure 10. Conductometric titration for Co(OAc)2-HNB system.
![]()
Figure 11. Conductometric titration for Co(OAc)2-HNT system.
in solution and hence we could obtain each of them by adding the appropriate amount of the ligand. Fe3+-HNB curve (Figure 11) revealed only one break at 1:2 (Co2+:HNT).
A common behavior of these curves is the continuous decrease of the reaction mixture conductance with increasing the number of hydrazones added. This suggests the formation of non-electrolytic species in solution, i.e., the reaction of HNB or HNT with Co2+ acetate proceeds without the liberation of acetic acid. Conversely, Co2+-HNB, HNT complexes were isolated as solids by deprotonation of ligand and thus the acetic acid was liberated. This can be interpreted on the basis of various reaction conditions. To explain this contrariety, we proposed that HNB or HNT reacts with cobalt acetate in solution and at room temperature forming an octahedral geometry around the Co2+ ion. Upon refluxing the reaction mixture, the liberation of acetic acid is induced and finally, a non-electrolytic complex species having a square-planar geometry is obtained as in the solid-state. This mechanism can be shown in case of HNB ligand as follow:
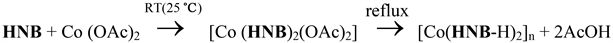
The same mechanism is proposed for the Co2+-HNT complex. Finally, the molar conductivity of all complexes is shown in Table 1. The values indicate a non-electrolytic nature in this solvent and are consistent with the other results.
4. Conclusion
The results obtained in this work verified that a number of aroyl hydrazones complexes, derived from 2-hydroxy-1-naphthaldehyde, have been successfully synthesized. The compositions and structures of these complexes have been established on the basis of different physicochemical techniques. The mode of binding of the aroyl hydrazones under investigation mainly depends on the substitute in the para-position of the phenyl ring. The persistence of the electron-donating group in the para-position of phenyl ring e.g., methyl group increases the electron density on azomethine nitrogen and consequently facilitates its coordination with the metal cation. This is very conspicuous in the solid complexes of aroyl hydrazones with cobalt, nickel, and copper ions. The chelating takes place with the replacement of phenol hydrogen along with the linking of an auxiliary group that is SO2 in HNB and C=N in HNT forming complexes with a ratio of 1:2 (M: L) composition for HNB and polymer chains with HNT. Spectrophotometric and conductance titration studies can be used to identify the stoichiometry of the aroyl hydrazones with some metal cations and the former technique substantiates the possibility of micro determination of Co2+ and Fe3+ ions by the aroyl hydrazones.