Pd-Catalyzed Unprecedented Cross-Coupling of Mixed Phenols and Halides for the Synthesis of Aromatic Ethers ()
1. Introduction
Cross-coupling becomes a common term in organic chemistry in number of different reactions namely, two different fragments are joined together with the support of a metal catalyst. Carbon-oxygen is one of the most effective bond formation reactions like C-C bond formation in organic chemistry. Cross coupling process is essentially equally applied to explore C-O bond formation reactions [1] - [10].
In 2020, after a series of tests that conducted by Al-Masum group found palladium complex, PdCl2(dppf)CH2Cl2, an effective catalyst for C-O bond formation for cascade type multiple phenolic ether synthesis in one step [11]. Therefore, this project aims to extend the earlier findings of phenolic ether synthesis in investigating the possibility of making aromatic ethers from mixed phenols and halides in the presence of PdCl2(dppf)CH2Cl2 under microwave irradiation.
2. Results and Discussion
The diaryl ethers are found in a great number of natural products and synthetic pharmaceuticals [12] [13] [14]. They are found in many pesticides, polymers, and ligands. In 1905, Ullmann’s reaction of phenols with aryl bromides in presence of KOH to form biaryl ethers. However, the procedure for biaryl ether formation requires stoichiometric amount of copper reagents and high temperatures (typically > 160˚C).
After a series of attempts of reactions using different ratios of starting materials, reaction times, and temperature levels, optimized reaction conditions were established that were used to synthesize variety of phenolic ethers (Figure 1).
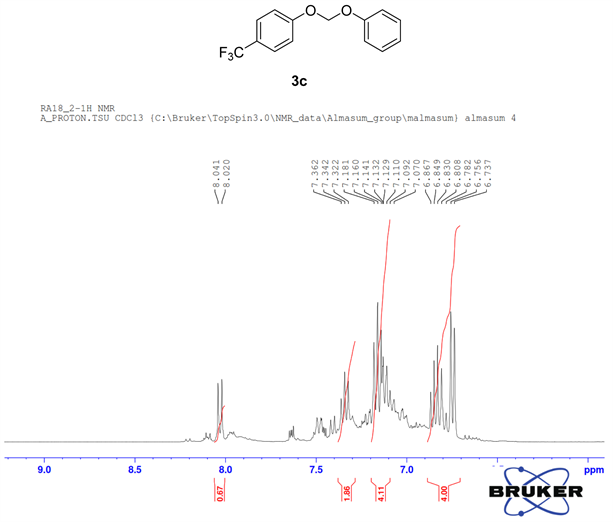
In order to investigate further application of PdCl2(dppf)CH2Cl2, in cross-coupling reactions of mixed phenols with bromo iodomethane 2a, chloro iodomethane 2b, 1,2,4-tribromobenzene 2c were attempted. After running several reactions with different temperature levels and duration times, the cross-coupling of chloro iodomethane 2b showed good results when interacting with mixed phenols 1b and 1c in the presences of PdCl2(dppf)CH2Cl2 (Figure 4, product 3c). In this reaction, we used 0.5 mmol of phenols 1b and 1c with 0.5 mmol of chloro iodomethane 2b. The reaction vial was in the microwave oven for 5 hours at 100˚C. The phenolic ether, 3c was obtained in 65% yields.
The formation of ether 3c by cross-coupling reaction involved 0.5 mmol (81 mg) of phenol 1b and 0.5 mmol (48 mg) of phenol 1c and 0.5 mmol (36 µL) of chloro iodomethane 2b. The reactants were loaded in dry clean microwave vial and 2.00 mmol (195 mg) of NaO tBu, 5 mol% of PdCl2(dppf)CH2Cl2 (20.0 mg) were added to the mixture, then it was capped with septum and flushed with argon followed by the addition of 2 mL 1,4 Dioxane as solvent. The resulting mixture was irradiated at 100˚C for 5 h. For purification, the crude mixture was passed through alumina column chromatography using hexane/ethyl acetate (92%/8%) as eluents. The essentially pure product 3c was collected.
The cross coupling of bromo iodomethane 2a gave the desired products when reacted with phenols in the presence of 1,4-dioxane as a solvent. To furnish this
![]()
Figure 1. Phenolic ether from choloro iodomethane.
phenolic ether product, 0.5 mmol (66 µL) of phenol 1a and 0.5 mmol of phenol 1c (48 mg), 0.5 mmol of bromo iodomethane, 5 mol% of PdCl2(dppf)CH2Cl2, and 2 mmol of NaOtBu were used. The reaction vial was in the microwave oven for 1 h at 125˚C (Figure 2). In Figure 4, it is shown as a product 3b in 72% yields.
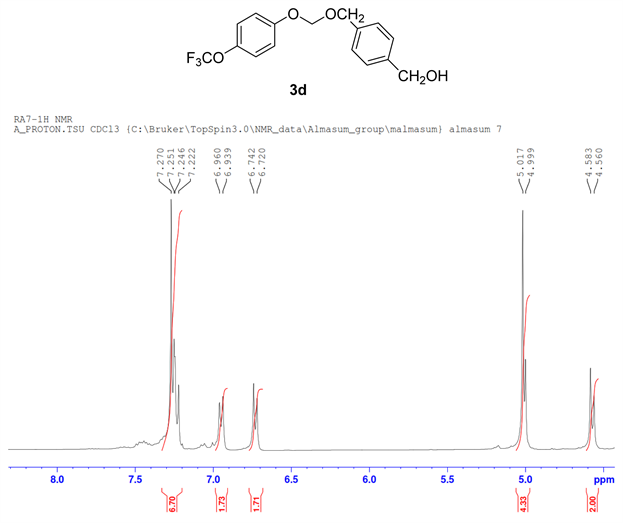
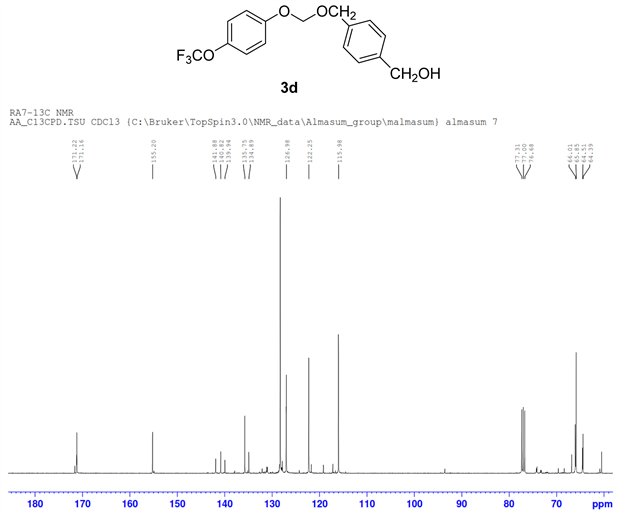
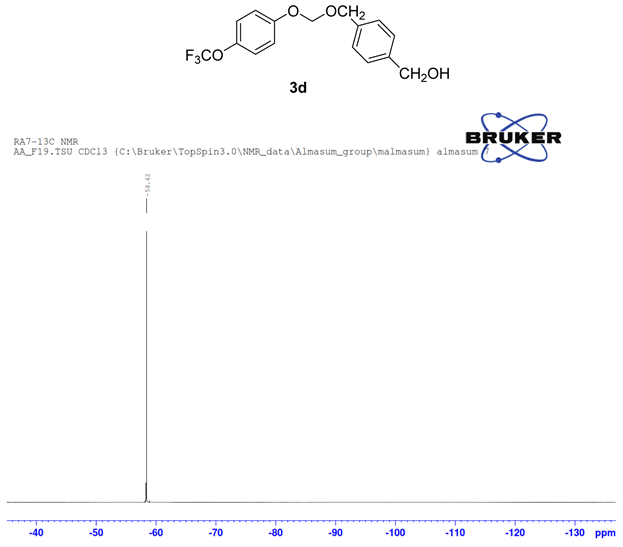
The formation of ether 3d by cross-coupling reaction involved 1.00 mmol (138 mg) of 1,4-benzenedimethnol 1d, 0.5 mmol (66 µL) 4-trifluoromethoxy phenol 1a and 0.5 mmol (40 µL) of bromo iodomethane 2a. The reactants were loaded in dry clean microwave vial and 2.00 mmol (195 mg) of NaO tBu, 5 mol% of PdCl2(dppf)CH2Cl2 (20.0 mg) were added to the mixture, then it was capped with septum and flushed with argon followed by the addition of 2 mL of 1,4 Dioxane as solvent. The resulting mixture in vial was irradiated at 80˚C for 5 h. The crude reaction product in reaction vial diluted with ethyl acetate was transferred into a separatory funnel. Water was then added to the funnel and after standard extraction, excess alcohol was miscible with aqueous layer and drained. The remaining organic layer in the separating funnel was collected in a small Erlenmeyer flask over anhydrous Na2SO4. The ethyl acetate layer was filtered through sintered funnel and collected filtrate in a round bottom flask was completely dried by rotary evaporator in vacuo. The column chromatography technique was used to sperate the product. The desired mixed phenolic ether product 3d (Figure 4) was confirmed by 1H NMR, 13C NMR, and 19F NMR (Figure 3).
NMR data of products from Figure 4, product 3a, 3b, 3c, 3d, 3e, and 3f are given below.
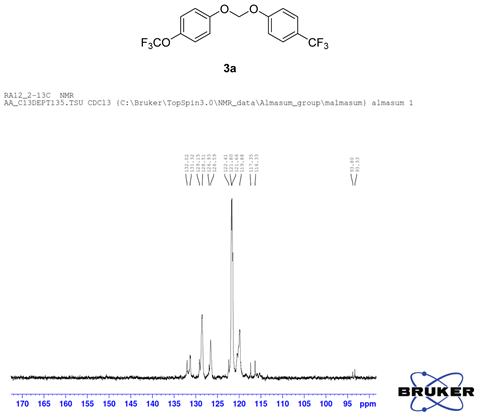
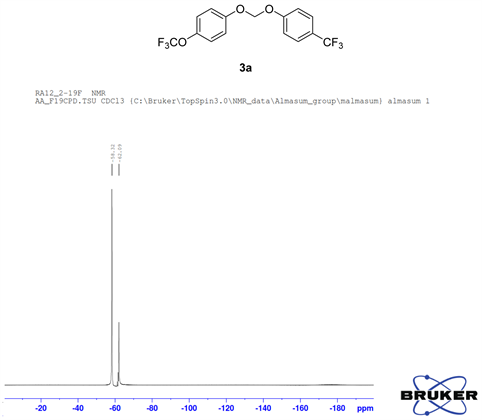
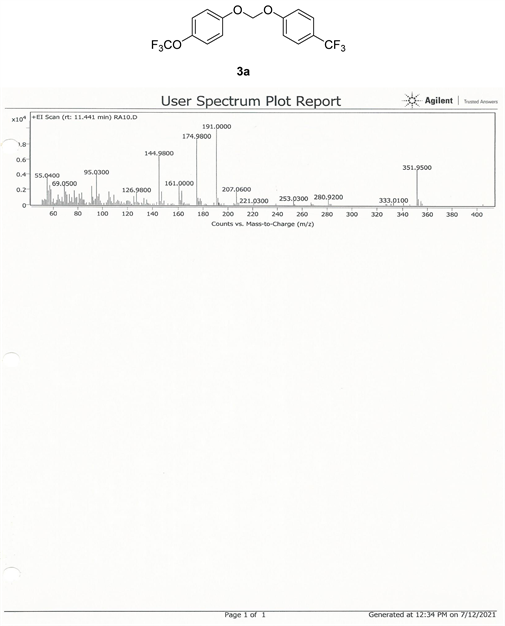
Compound 3a, 13C NMR (CDCl3, 100 MHz) δ 158.3, 155.5, 130.7, 129.5, 128.2, 122.2, 120.5, 116.4, 115.2, 93.5; 19F NMR (CDCl3 400 MHz) δ −58.3 (OCF3), −62.0 (CF3); LRMS: Calc’d for C15H10O3F6(M+): 352. Found: 351.95.
![]()
Figure 2. Cross-coupling of bromo iodomethane and phenols.
![]()
Figure 3. Phenolic ether from mixed phenol.
![]()
Figure 4. Phenolic ethers from mixed phenols and halides. All products are collected by passing the crude reactions products through alumina column using the eluents, hexane 92%/ethyl acetate 8%. Yields of the products are ranges from 70% to 60%.
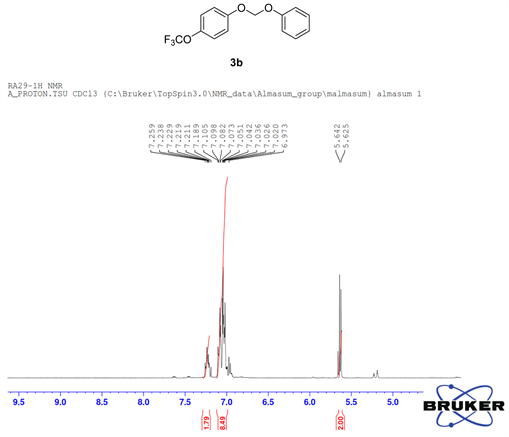
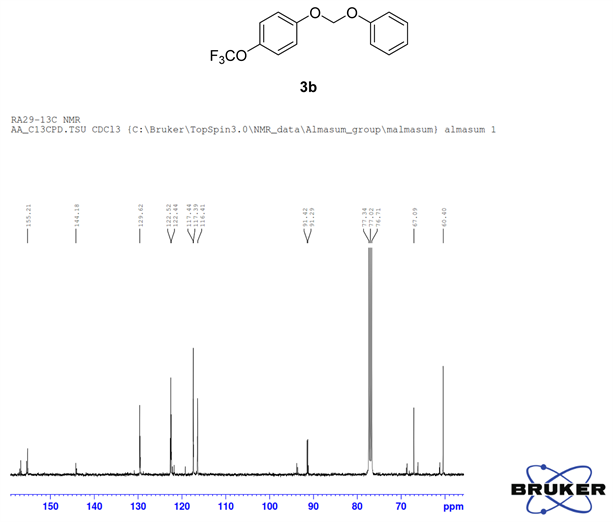
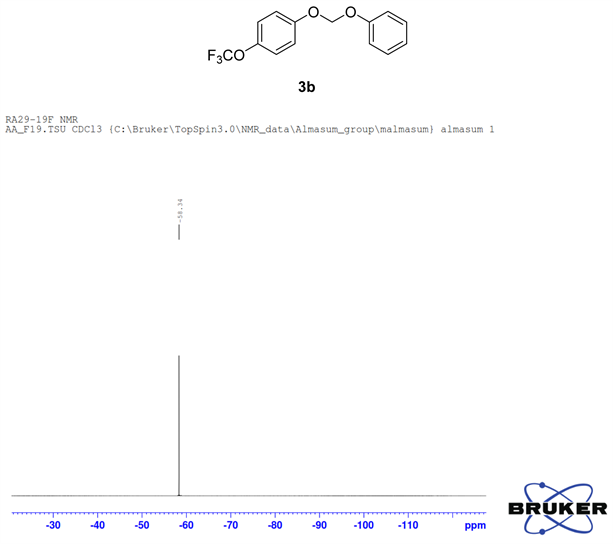
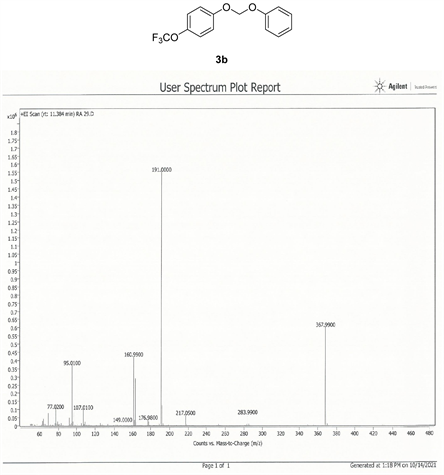
Compound 3b, 1H NMR (CDCl3, 400 MHz) δ 7.25 - 6.97 (m, 9H,), 5.63 (m, 2H); 13C NMR (CDCl3, 100 MHz) δ 155.2, 144.1, 129.6, 122.5, 122.4, 117.4, 117.3, 116.4, 91.3; 19F NMR (CDCl3 400 MHz) δ −58.3 (OCF3); LRMS: Calc’d for C14H11O3F3(M+): 284. Found: 283.99.
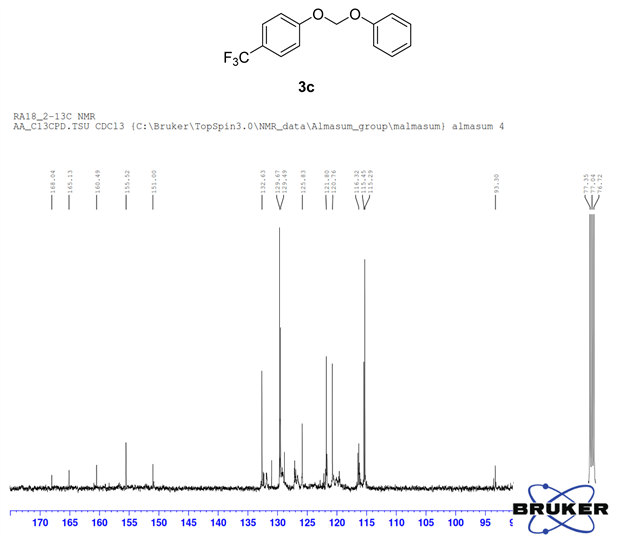
Compound 3c, 1H NMR (CDCl3, 400 MHz) δ 8.04 - 6.78 (m, 9H), 6.74 (m, 2H); 13C NMR (CDCl3, 100 MHz) δ 165.1, 160.4, 155.5, 151.0, 132.6, 129.5, 125.8, 121.8, 121.7, 115.2, 93.3; 19F NMR (CDCl3 400 MHz) δ −61.5.
Compound 3d, 1H NMR (CDCl3, 400 MHz) δ 7.20 - 6.72 (m, 8H), 5.01 (m, 2H), 4.4.99 (m, 2H), 4.57(m, 2H). 13C NMR (CDCl3, 100 MHz) 155.2, 141.8, 139.9, 135.7, 128.5, 126.9, 122.2, 115.9, 66.0, 64.5; 19F NMR (CDCl3 400 MHz) δ −58.4.
Compound 3e,1H NMR (CDCl3, 400 MHz) δ 7.37 - 6.69 (m, 5H, 2H); 13C NMR (CDCl3, 100 MHz) δ 155, 130.2, 129.5, 121.5, 120.5, 116.4, 115.293.5.
Compound 3f, 1H NMR (CDCl3, 400 MHz) δ 7.69 - 7.20 (m, 17H); 13C NMR (CDCl3, 100 MHz) δ 136.0, 134.6, 131.6, 125.8, 123.7, 121.3; 19F NMR (CDCl3 400 MHz) δ −61.9.
3. Conclusion
In conclusion, this project extends the earlier findings of phenolic ether synthesis in investigating the possibility of making aromatic ethers from mixed phenols and halides in the presence of PdCl2(dppf)CH2Cl2 under microwave irradiation. The research paves a continuing pathway to achieve a new method for establishing polyphenolic ethers from phenols and halides. As this project obtained some success and builds upon previous studies, hopefully, it reaches new boundaries for creating novel series of polyphenolic ethers important bioactive sources for scientists interested to explore human health benefits and prevent diseases. Each step of this project was challenging because of the adjustments per small reaction scale. The separation techniques didn’t perform well in some reactions which affected the final amount of the product yields.
Supporting Information
The experiments used a CEM microwave system. Also, a Varian 3900 model used to analyze GC-MS. 1H NMR, 13C NMR, and 19F NMR spectra were recorded by Bruker 400 MHz NMR. All the data of GC-MS and NMR spectra are shown in the appendix. All the solvents that used in the experiments were purchased through Fischer Scientific, Sigma-Aldrich with dry condition, and in Sure-sealed bottled. Also, the palladium catalysts used were purchased through Alfa Aesar. All the reagents used were purchased from Sigma-Aldrich.

![]()
![]()
![]()
![]()
![]()
![]()
![]()