Mechanical Heart Valves and Pregnancy to Patients Admitted at Jakaya Kikwete Cardiac Institute, Dar es Salaam-Tanzania ()

1. Introduction
Normal pregnancy is associated with 30 - 50 percentage increase blood volume and CO. These increases begin during the first trimester, the levels peak by 20 to 24 weeks of pregnancy and then are either sustained until term or decrease. The heart rate increases by 10 to 20 beats per minutes, the stroke volume increases, and there is a substantial reduction in systemic vascular resistance, with decreases in blood pressure [1] [2].
During labour, cardiac output increases and the blood pressure also increases. Immediately after delivery, the cardiac filling pressure may increase dramatically due to the decomposition of the venacava and the return of uterine blood. The cardiovascular adaptions associated with pregnancy regress by approximately six weeks after delivery. The functional murmurs develop in nearly all women during pregnancy. Echocardiography is warranted when diastolic murmurs, continuous murmurs, or loud systolic murmurs (louder than grade 2 on the 6 point scale) are detected or when murmurs are associated with symptoms or an abnormal electrocardiogram [3] [4].
The development of the original ball-and-cage valve design can be attributed to the bottle stopper in 1858. In early 1950’s, it led to the idea of a prosthetic heart valve, first implanted in human in a closed procedure in September of 1952 by Charles Hufnagel, who implanted heterotopic valvular heart prosthesis in the descending aorta of a patient with aortic valve regurgitation. This represented the first step in a long journey that lasted for more than 50 years and not finished yet. Heart valve innovation has been one of the most important factors influencing the evolution of cardiovascular medicine. It is also a good example of team work between physicians and engineers [1] [3] [5].
2. Methods
A retrospective hospital-based matched cohort study was conducted at Jakaya Kikwete Cardiac Institute (JKCI), Dar es Salaam-Tanzania. Included were all women of child-bearing age who had bio prosthetic or mechanical replacement, this method ensured accuracy of the clinical study documents from prior studies which were cross-checked with the new data. This process involved multiple steps and cross checks to ensure the completeness and accuracy of all data. The study design is important because the exposure to risk factors is recorded before the occurrence of the outcome, which allows the temporal sequence of risk factors and outcomes to be assessed; hence it permitted the epidemiology of mechanical heart valves to be studied.
3. Results
Between January 2018 and April 2021 a total of 600 patients received a speciality care during pregnancy. There were more than 50 live births among 22 women with the mechanical prosthetic valves and more than 500 live births among the 367 women in the matched community comparison group who were created by non-random method during the study. Severe maternal morbidity occurred in 20 pregnancies (13.3%) in the mechanical prosthetic valve group and 9 (6.0%) in the community comparison group. There were no maternal deaths. The prolonged hospital lengths of stay were high due to complications that the patients underwent during treatment.
4. Discussions
Mechanical valves have excellent durability and hemodynamic profile. Mechanical prosthetic valves, however, are pregnant women with mechanical heart valves require careful, adequate anticoagulation with frequent monitoring. Warfarin provides better maternal protection against thromboembolism but may be harmful to the foetus. Heparin is less protective against maternal thromboembolism but is safer for the foetus. Other physiological changes are such as; hyper-coagula state, hypo-albumineania, insulin resistant state, increased red cell mass, increased ESR, increased renal blood flow (30%) and increased hepatic clearance of medications [6] [7] [8].
The etiology of thrombosis in pregnancy can be due to an increased serum levels of procoagulants, fibrinogen and factors II, VII, VIII, X and XII, the decreased protein S levels, an increased resistance to activated protein C second and third trimesters of pregnancy, venous stasis and an increased serum plasminogen activator inhibitor-1 (PA1-1) and placental PA1-2 levels [9] [10] [11] [12] [13]. The risks that can result from thrombosis include a history of a prior thromboembolic event, atrial fibrillation, prosthesis in the mitral position, multiple prosthetic valves, mechanical prosthetic valves and pregnancy [14].
Moreover, patients with valvular heart can poorly tolerate, Aortic stenosis, mitral regurgitation, aortic regurgitation with New York Heart Association (NYHA) class 2 - 4 symptoms, mitral stenosis with NYHA class 2 - 4 symptoms, Valvular heart disease that results in severe pulmonary hypertension, left ventricular (LV) dysfunction with an ejection fraction(EF) less than 40%. Figure 1 below elaborates more about the complications of mechanical valves [8] [11].
4.1. Anticoagulation Therapy
These medicines are given to prevent the blood from clotting as quickly or as effectively as normal. They can be classified into parenteral anticoagulants and Oral anticoagulants, whereas the parenteral anticoagulant involves the Indirect Thrombin Inhibitors and Direct Thrombin Inhibitors. The direct thrombin inhibitors is composed of hirudin, lepirudin, bivalirudin and argatroban, while indirect thrombin inhibitors are such as heparins which are of high molecular
![]()
Figure 1. Complications of mechanical valves.
weight heparins and low molecular weight heparins, such as; enoxaparin, tinzaparins, reviparin, danaparoid and dalteparin. Oral anticoagulants are of two types which are; coumarins which contain warfarin and dicumol, and indanediones which contain phenindione [3] [9].
4.2. Anticoagulation in Pregnant Patients with Valvular Heart Disease
Warfarin is more efficacious than unfractionated heparin (UFH) for thromboembolic prophylaxis of pregnant women with mechanical valves. Warfarin therapy in the first trimester of pregnancy is associated with a substantial increase in foetal anomalies [3] [9].
1) Foetal wastage (approximately 30%);
2) Prematurity (approximately 45%);
3) Low birth weight (approximately 50%).
The antidotes of warfarin include; Vitamin K (Antidote), Fresh frozen plasma, Prothrombin complex concentrates and Recombinant factor VIIa [11].
Heparin activates Anti Thrombin III, Monitoring , APTT for High MW Heparin and Anti X a level for LMWH (0.7 - 1.2 IU/ml), Half-life 1.5 hr, Excretion, Bile and Urine, Antidote, Protamine sulphate , 1 mg/100 units heparin [1].
4.3. Maternal Complications of Anticoagulants during Pregnancy
・ The rate of major bleeding in patients treated with UFH therapy is 2%;
・ Approximately 3% of patients receiving UFH develop HIT;
・ Heparin-induced osteoporosis causes vertebral fracture in 2% - 3% of patients;
・ Significant reduction in bone density in 30%;
・ LMWH causes less osteoporosis and HIT than UFH [13] [15].
4.4. Foetal Complications
・ Warfarin crosses the placenta and can cause foetal bleeding and teratogenicity especially in first trimester.
・ UFH & low LMWH do not cross the placenta.
Although bleeding at the uteroplacental junction and foetal wastage are possible [11] [13].
4.5. Regimens of Anticoagulant
・ Regimen 1-Warfarin sodium throughout pregnancy with unfractionated heparin sodium near term.
・ Regimen 2-Substitution of warfarin with unfractionated heparin between 6 - 12 weeks and near term [15].
・ Regimen 3-Unfractionated heparin throughout pregnancy [15].
4.6. Prosthetic Heart Valves and Anticoagulation
Regimen with lowest risk of valve thrombosis (3.9%) [1] [4] [14]
・ Warfarin throughout pregnancy.
・ At cost of warfarin embryopathy in 6.4% of live births.
・ This risk was eliminated when heparin was substituted for warfarin at or prior to 6 weeks and continued until 12 weeks.
・ Although using heparin only from 6 - 12 weeks’ gestation was associated with an increased risk of valve thrombosis (9.2%).
4.7. Problems That May Occur in Pregnancy
Foetal complications that may occur as a result of anticoagulation therapy miscarriage, growth, restriction, prematurity and low birth weight [13]. Graph 1 below illustrates more.
Maternal complications that may occur include the increase risk of valve thromboembolism, 25% risk of significant maternal morbidity such as myocardial infraction and stroke, estimated 3% risk of maternal mortality [10] [13]. Graph 2 below illustrates more details.
4.8. Treatment
・ Warfarin should be stop before 06 weeks of pregnancy [4] [6] [11].
・ 06 - 13 weeks Heparin.
・ 13 - 36 weeks Warfarin.
・ 36 weeks to birth Heparin.
Heparin should be stop 06 hours before birth and resume 06 hours after birth and continue for 24 to 72 hours then switch to warfarin [4] [6] [11].
4.9. Treatment of Stuck Valve
・ Heparin may be considered for small, non-obstructive thrombi.
・ For obstructive valve thrombosis, the treatment options are surgical thrombectomy and thrombolysis, both of which carry substantial foetal and maternal risks.
・ Cardiac surgery during pregnancy, Perioperative maternal and foetal mortality was 6 and 30% respectively.
・ Thrombolysis during pregnancy maternal mortality, haemorrhagic complications, and foetal mortality rates were 1.2, 8, and 5, 8% respectively [7] [11] [12] [13] [14].
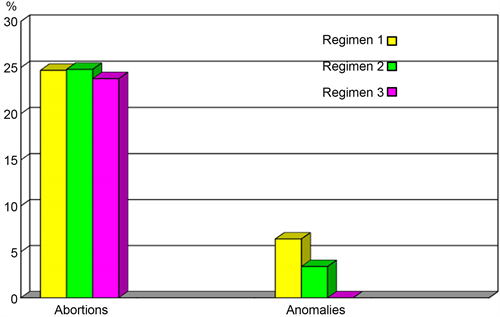
Graph 1. A graph representing foetal complications.
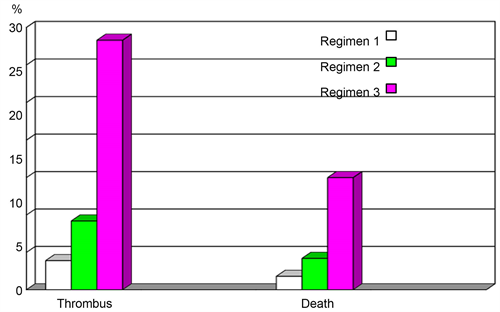
Graph 2. A graph representing maternal complications.
5. Conclusions
Pregnant women with mechanical prosthetic valves were more likely to experience severe maternal morbidity, as well as prolonged hospital length of stay, than matched counterparts without heart disease. This information can enhance shared decision making about the timing of valve replacement and pregnancy planning in young and middle-aged women.
Moreover, pregnant women with mechanical heart valves require careful, adequate anticoagulation with frequent monitoring. Warfarin provides better maternal protection against thromboembolism but may be harmful to the foetus. Heparin is less protective against maternal thromboembolism but is safer for the foetus.
Acknowledgements
I would like to pass my special appreciation to the Management of Jakaya Kikwete Cardiac Institute for granting me a permission to conduct this study, not only for those mentioned but also great gratitude to all staffs of internal medicine, gynaecology department, and laboratory for their support during data collection.
I would like to thank all patients, without them this study could not have been possible.
Last but not least I would like to thank my team particularly my personal secretary, Ms Bethsheba Sakinoy for the good work, also special gratitude to government for their support.
Availability of Data and Materials
The datasets analysed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval
The review was conducted after approval by the joint ethical research committee of the Jakaya Kikwete Cardiac Institute, administration particularly department of gynaecology and obstetrics, community medicine and research.
List of Abbreviations
ECG―Electrocardiography; EF―Ejection Fraction; HMWH―High Molecular Weight Heparins; JKCI―Jakaya Kikwete Cardiac Institute; LMWH―Low Molecular Weight Heparins; LV―Left Ventricular; SC―Subcutaneous; UFH―Unfractionated Heparin.