Effect of Mn on the Performance and Mechanism of Catalysts for the Synthesis of (Ce,La)CO3F ()
1. Introduction
In recent years, domestic coal consumption has tended to increase and the air pollution problem caused by the coal combustion process has become increasingly serious, with coal-fired power plants representing 60% of the overall emissions from stationary sources. The use of coal, diesel and petrol to produce NOx can lead to a range of environmental problems, so NOx removal and reduction is imperative. SCR technology (Selective Catalytic Reduction) is now favoured by many researchers in the field of denitrification. The traditional V2O5-WO3(MoO3)/TiO2 catalyst has not yet been solved due to its toxicity and narrow denitration temperature window, which has led to the research of new catalysts. Mineral catalysts have received increased attention from scholars due to their non-toxicity and wide range of elements.
The main rare-earth minerals in Baiyun Ebo rare-earth concentrates are bastnaesite, and the main rare-earth elements in rare-earth concentrates are Ce, La, Nd and Pr [1], of which cerium has a very efficient and promising future as a mineral catalytic material. However, the composition of rare earth concentrates is complex and the available characterisation tools have no way of determining the specific reaction changes of a multi-phase component catalyst. Exploring its specific reaction mechanism as a catalyst is also a blind spot that is difficult to break through, so we need to look at the mineral phases that play a catalytic role in the concentrate on a case-by-case basis and explore their denitrification mechanisms. As the main mineral phase in rare earth concentrates, the study of bastnaesite is necessary. The present technical means cannot extract the more pure bastnaesite, but there are many scholars who use the pure material to synthesise the mineral to study its properties. Huang Shunhua et al. [2] synthesised bastnaesite by hydrothermal method. Experimental results showed that from room temperature to 400˚C, atmospheric pressure to 100 MPa, the solution pH from 6.7 to 11.0 range as long as the necessary substances to form the mineral can be synthesized bastnaesite. The molecular formula of bastnaesite is (Ce,La)CO3F according to the mineralogy of the Baiyun Ebo rare earth concentrate process, and the (Ce,La)CO3F crystals were prepared by the hydrothermal method of analytical purity.
The roasting of (Ce,La)CO3F produced Ce7O12 species, which had poor performance as denitrification catalysts due to its good crystallinity, so the catalytic performance of (Ce,La)CO3F was promoted by introducing the transition metal Mn to improve the dispersion of Ce7O12 species, as well as generating new active components. Cerium-based catalysts have excellent low-temperature catalytic performance, and many researchers have used CeO2 as a carrier to improve the performance of NH3-SCR. Solid oxide catalysts are sourced for due to their advantages ranging from low cost, recoverability and reusability, environmental benign-ness, thermal stability and high quality product generation [3]. Yao Xiaojiang [4] prepared a series of MnOx/CeO2 catalysts by adjusting the solvents (deionised water, anhydrous ethanol, acetic acid, oxalic acid solution). The MnOx/CeO2 catalysts prepared with oxalic acid solution as the solvent showed over 80% NO conversion in the range of 100˚C - 250˚C, and good low temperature sulphur and water resistance, probably because the solvent oxalic acid enhanced the electronic interaction between MnOx and CeO2 and increased the oxygen vacancies in the carrier CeO2, which can promote the decomposition of NO species. Therefore, this paper is used to improve the catalytic performance of (Ce,La)CO3F by loaded Mn, and to study its NH3-SCR physicochemical properties and reaction mechanism by characterization and in-situ infrared, to clarify the specific reaction mechanism within bastnaesite, and to provide theoretical guidance for the reaction performance and mechanism of rare earth mineral catalysts.
2. Experimental Methods
2.1. Experimental Materials
Reagents used in the experiments, Ce(NO3)3·6H2O (mass fraction), analytical purity, Tianjin Comio Chemical Reagent Co. La(NO3)3·6H2O (mass fraction), analytical purity, Tianjin Comio Chemical Reagent Co. NaHCO3, analytical purity, Tianjin Windship Chemical Reagent Technology Co. NaF, analytical purity, Tianjin Windship Chemical Reagent Technology Co. Mn(NO3)2, analytical purity, Shanghai Zhangyun Chemical Co.
2.2. Preparation of Catalyst
The synthesis of (Ce,La)CO3F was carried out by hydrothermal method. A certain amount of Ce(NO3)3·6H2O, La(NO3)3·6H2O, NaF, NaHCO3 was placed in 100 ml of PTFE liner at room temperature. Then 80 ml of distilled water was poured into the PTFE liner with constant stirring, and the PTFE liner was placed into an autoclave under atmospheric pressure and 120˚C with stirring and heating for 2 h for hydrothermal reaction. After cooling, the mixture was filtered and dried at 80˚C to obtain synthetic (Ce,La)CO3F. After cooling, the mixture was filtered and dried at 80˚C to obtain synthetic (Ce,La)CO3F. Mn(NO3)2 was dissolved in deionised water using the impregnation method and continuously stirred to form manganese nitrate solutions at concentrations of 0.2 mol/L, 0.4 mol/L, 0.6 mol/L, 0.8 mol/L and 1.0 mol/L, respectively. The synthetic (Ce,La)CO3F was used as a carrier and poured into different concentrations of Mn(NO3)2 solution, sonicated for 2 h, left overnight, filtered and dried the suspension, and then roasted in a muffle furnace at 500˚C for 2 h. The solid material obtained was the Mn/(Ce,La)CO3F catalytic material.
2.3. Experimental Procedure
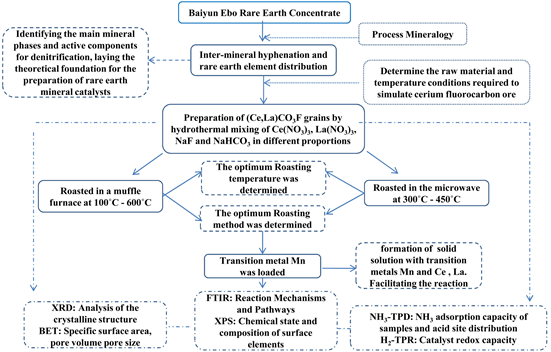
2.4. Testing of Catalytic Performance
The experiments were carried out in a reaction apparatus with quartz tubes for testing the activity of the catalyst NH3-SCR. The reaction apparatus consists of a gas mixing tank-flow meter, standpipe furnace, quartz tube, Fourier infrared spectroscopy flue gas analyser and computer data acquisition system. The standpipe furnace was heated by a silicon-molybdenum rod model 1800 with a rated temperature of 1600˚C and an internal diameter of 20 mm and a length of 1.2 m from Nanjing Boynton Instrument Technology Co. The Fourier infrared spectroscopy (FTIR) flue gas analyser and data acquisition system were manufactured in Finland, and the model number was GASMET-DX4000. The simulated gas components were as follows: NH3 500 ppm, NO 500 ppm, O2 at a volume fraction of 6% of the total, N2 as the equilibrium gas, a total gas flow of 100 ml/min, an air velocity of approximately 8000 h−1·g−1 and a catalyst dosage of 0.6 g for each test.
3. Results and Discussion
3.1. Catalytic Performance Tests
Figure 1 showed the NOx conversion and N2 selectivity of the Mn/(Ce,La)CO3F
![]()
Figure 1. NOx conversion over catalyst (a) N2 selectivity (b). (Reaction conditions 500 ppm NO, 500 ppm NH3, 6% O2, N2 as balance gas).
catalysts obtained with Mn loaded. From the denitrification activity results, it can be seen that the NOx conversion of the Mn/(Ce,La)CO3F catalysts with different loadings all increased with temperature in the temperature range of 100˚C - 150˚C, but as the temperature continued to be increased, the NOx conversion started to decrease at 200˚C and then increased again at 250˚C to reach the highest value for the whole catalytic reaction.
The reason for this may be due to the large amount of NO adsorbed on the catalyst surface in the range of 100˚C - 150˚C, which reduces the value of NOx, but NOx is not being reacted on the catalyst surface at this point, merely occupied as an active site, and as NO continues to pass through, NO cannot continue to be adsorbed, due to the absence of active sites on the catalyst surface. It is not until 250˚C that the NH3-SCR reaction on the catalyst surface begins to take place, allowing the catalyst to reach maximum denitrification efficiency. It may also be due to the activation of species by adsorption of NH3 and NO on the catalyst surface. That is to say, NH3/
and species such as NO2, nitrate and nitrite are less stable on the catalyst surface and are particularly susceptible to temperature, decomposing at 200˚C, which leads to a decrease in activity. It can be found that when (Ce,La)CO3F is impregnated in a 1 mol/L solution of Mn(NO3)2, the conversion of catalyst NOx can reach 80% at 250˚C. However, as the temperature continued to increase, the conversion rate also gradually decreased, which may be due to the oxidation of the reducing agent NH3, which resulted in a decrease in the amount of reducing agent, and the oxidation of NH3 at high temperature would release NO, which increased the concentration of NO but decreased the conversion rate. Figure 1(b) showed the N2 selectivity graphs for the synthesis of (Ce,La)CO3F as well as (Ce,La)CO3F loaded with Mn. N2 selectivity is another important indicator to evaluate denitrification performance. In the NH3-SCR denitrification process, side reactions such as oxidation of ammonia and high temperature decomposition of nitrate and ammonium nitrogen lead to partial production of N2O, which greatly reduces the N2 selectivity. From Figure 1(b) it can be obtained that when loaded with the transition metal Mn, the N2 selectivity of the catalyst starts to decrease after 150˚C, which is related to the excessive hydrogen capture due to the strong redox ability of Mnn+.
3.2. Physical Phase Structure Analysis
Figure 2 showed XRD diagrams of the synthetic (Ce,La)CO3F catalyst roasted at 200˚C -600˚C in a muffle furnace. As can be seen in Figure 2, at 200˚C most of the CeCO3F, LaCO3F and (Ce,La)CO3F do not decompose, but the degree of crystallinity and dispersion is greatly improved and many of the diffraction peaks are significantly reduced.
At 300˚C the diffraction peaks of species such as CeCO3F and LaCO3F are further reduced and some decomposition occurs. The decomposition of CeCO3F gave rise to Ce11O20, Ce6O11, Ce7O12 and CeF3 species, and the decomposition of LaCO3F gave rise to LaCO, La2O3 and LaF3 species, with the (Ce,La)CO3F catalyst starting to decompose at 300˚C. At 400˚C, the most active stage of decomposition on the catalyst surface, CeCO3F and LaCO3F species were completely decomposed, with only a few species in amorphous form on the catalyst surface, and a large amount of CeCO3F decomposed into Ce11O20 and Ce6O11 species, and Ce7O12 species increased significantly compared to 300˚C. The main diffraction
![]()
Figure 2. XRD patterns of (Ce,La)CO3F after roasting at different temperatures.
peaks were mostly a composite of Ce7O12 and Ce6O11 species, both of which were formed under very similar conditions. The 500˚C roasting condition is the most stable stage for the catalyst surface species, and the main diffraction peaks of the catalyst are all Ce7O12 species, while LaCO3F is present as La2C2O2, with some CeF3 and LaF3 species also present. Combined with the activity tests at different roasting temperatures, the best denitrification effect was obtained after roasting at 500˚C. Therefore, combined with the XRD pattern at 500˚C, it can be seen that the Ce7O12 species is the main active component of the reaction, which is more favourable to interact with other substances to promote the reaction than the Ce11O20 and Ce6O11 species. At 600˚C, the Ce7O12 species on the catalyst surface decreased and Ce11O20 and Ce6O11 species gradually appeared, so there was a relative decrease in the active component, perhaps due to the change in catalyst structure caused by the high roasting temperature, which was not conducive to the reaction.
Figure 3 showed the XRD patterns of Mn loaded by the over-impregnation method. From Figure 3, it can be seen that the diffraction peaks of Ce7O12 were dominated on the catalyst surface, accompanied by some CeO2 species produced after roasting, and CeOF species. The diffraction peaks of Ce7O12 species on the catalyst surface decreased gradually with increasing loading. Indicated that the addition of manganese nitrate can cause more cracks and oxygen vacancies on the surface of the carrier, which is conducive to improving the dispersion of the active components on the surface of the carrier, and Mn can interact with Ce and La in the synthetic (Ce,La)CO3F to reduce the crystallinity and increase the specific surface area. The La2O2C2 species also showed some improvement in
dispersion with increasing Mn loading, and from the figure, MnOx species such as Mn3O4, Mn2O3, MnO2 and MnO can also be observed. There were more MnOx species on the 0.8 mol/L and 1 mol/L catalysts compared to the less loaded catalysts. The presence of MnOx species is beneficial to the improvement of catalytic oxidation reduction, and the dispersion of MnOx species on the catalyst gradually became better, mainly in amorphous form on the catalyst surface, and the amorphous structure was favourable to the improvement of catalytic performance [5]. Due to the multiple valence states of Mnn+, the interconversion of manganese ions facilitates the redox performance of the catalyst as well as the oxygen migration, both of which are beneficial for the conversion of NO to NO2. Generally speaking too high a loading will lead to a decrease in NO removal, this is because the dispersion of the transition metal in the catalyst starts to decrease, which leads to a conversion of the active material in the catalyst from an amorphous form to a crystalline form. It can be found that the highest catalytic activities of the catalysts with 0.8 mol/L and 1 mol/L Mn loading amounts are similar and basically close to each other. This indicates that the dispersion of Mn within the catalyst has tended to decrease. However, due to the poor redox and NH3 adsorption capacity of synthetic (Ce,La)CO3F, the synthetic (Ce,La)CO3F can be loaded with more Mn than other carriers and maintain a stable denitrification effect. It can also be observed from the figure that composite peaks of Ce7O12 species and MnOx species appear at 36.8˚, 38.5˚ and 55˚ in a companion relationship, which indicates that a Mn-Ce solid solution may have formed on the catalyst surface. In support of this conclusion, calculations of the cell constants were carried out for the Mn-loaded catalysts and the results are shown in Table 1. Compared with the synthesis of (Ce,La)CO3F, the crystal plane spacing and grain size of the catalyst decreased after loading Mn, and it was not until the crystal plane spacing increased that the surface structure of Mn/(Ce,La)CO3F was the most stable. The decrease in lattice parameters indicates that some of the Mnn+ has entered the lattice of (Ce,La)CO3F to form a Mn-Ce-La solid solution, due to the ionic radii of Mnn+ being Mn2+ (0.65 Å), Mn3+ (0.58 Å), Mn4+ (0.53 Å), and Cen+ being Ce4+ (0.97 Å), Ce3+ (1.14 Å), and La3+ (1.16 Å) in (Ce,La)CO3F. Since the ionic radii of Mnn+ are all smaller than Ce and La ions, the smaller ionic radii can replace the larger ones. When Ce4+ (0.97 Å), Ce3+ (1.14 Å) and La3+
![]()
Table 1. Cell constants of catalysts.
(1.16 Å) are replaced by Mnn+ with smaller ionic radii, it will lead to changes in the lattice parameters. In addition, the grain size of Mn-doped (Ce,La)CO3F is much smaller than when undoped due to the formation of Mn-Ce-La solid solution, which inhibits grain growth and causes lattice shrinkage, making the grain size smaller. It has also been reported in the literature that the formation of the Ce-M (metal ion)-O solid solution can inhibit the growth of metal oxide crystals and promote the activation of oxygen species, thus accelerating the NH3-SCR reaction [6].
3.3. Specific Surface Area Analysis
Figure 4(a) and Figure 4(b) showed the isothermal curves of N2 adsorption-desorption for different catalysts respectively, and Table 2 showed the specific surface area, pore capacity and pore size of each catalyst.
From Figure 4(a) it can be seen that the synthetic (Ce,La)CO3F catalysts exhibit a type II adsorption-desorption isotherm curve, such that the curve often occurs on non-porous solid surfaces or on macroporous solids free of a single
![]()
Figure 4. Adsorption-desorption isotherm profile of the catalyst. (a) (Ce,La)CO3F; (b) Mn(1.0 mol/L)/(Ce,La)CO3F.
![]()
Table 2. Catalyst specific surface area data.
multilayer reversible adsorption process. The curve is characterised by an inflection point at low P/P0, the first steep part of the isotherm, which indicated the saturated adsorption capacity of the monomolecular layer, and from Figure 4(a) it can be concluded that synthetic (Ce,La)CO3F has a poor adsorption capacity at low pressures. However, as the relative pressure increases, a second layer begins to form, and at saturation vapour pressure the number of adsorption layers is infinite, which also indicated a larger increase in adsorption capacity. The type II isotherm, commonly encountered at adsorbent pore sizes greater than 20 nm, has no upper limit to the solid pore size. In the low P/P0 region, the convex upward curve reflects a stronger interaction between the adsorbent and the adsorbate. In contrast, after doping with Mn, as shown in Figure 4(b), the Mn/(Ce,La)CO3F catalyst exhibits a Type III adsorption-desorption isotherm curve, which can be found to have no inflection point in the entire pressure range, and is convex downwards, often presenting this type when the adsorption interaction between the solid and the adsorbate is smaller than the interaction between the adsorbates. The small amount of adsorption in the low pressure region, and the absence of an inflection point, indicated that the forces between the adsorbent and the adsorbate were rather weak. The higher the relative pressure, the higher the adsorption amount, which showed a pore filling, which indicated that (Ce,La)CO3F has a rich pore structure when used as a carrier, which facilitates the entry and exit of the loaded metal ions and the reaction gas. As can be seen from Table 2, the physical structure of the carrier is affected by the loaded of the active component, and the specific surface area of the catalysts all decreased after loaded with the transition metal Mn, while the pore capacity and pore size increased. It has been reported in the literature that the pore size of the catalyst is in the mesoporous range, and that an appropriate increase in the pore radius of the catalyst facilitates the adsorption and desorption of the reacting gas molecules at the active sites on the catalyst surface, thus being more conducive to the catalytic reaction [7]. The increase in pore volume pore size is also due to the interaction of Mn, Ce and La to promote dispersion of each other, and facilitate the entry and exit of the reaction gases. However, it can also be found that the Mn/(Ce,La)CO3F catalyst made by impregnation in a 1 mol/L manganese nitrate solution has the best denitrification effect but not the largest specific surface area, which indicated that the specific surface area is only one of the factors affecting the catalytic activity and is not the main reason [8].
3.4. Redox and Adsorption and Desorption Performance Analysis
Figure 5(a) showed the NH3-TPD diagram of Mn/(Ce,La)CO3F catalyst obtained after ultrasonic impregnation of Mn loaded at different concentrations. Firstly, it can be seen from Figure 5(a) that the catalysts obtained by impregnation with 0.4 mol/L and 0.6 mol/L manganese nitrate solutions showed a similar trend in the desorption peak of NH3, and the catalysts obtained by impregnation with 0.8 mol/L and 1.0 mol/L manganese nitrate solutions have similar peak positions. Usually the binding of NH3 to the Brønsted acid site and the weak Lewis
![]()
Figure 5. NH3-TPD (a) H2-TPR (b) with different ratios of loaded Mn.
acid site is around 200˚C. The desorption peaks in the 400˚C range are caused by the desorption of ammonia at the moderately acidic site and the 500˚C peak is caused by the binding of ammonia to the strong Lewis acid site [9] [10]. The graph showed that the (Ce,La)CO3F sample showed a shoulder peak near 93˚C, where the desorption peak belonged to the binding of NH3 to the weak Brønsted acidic site, and at 312˚C, where the peak belonged to the binding of NH3 to the Brønsted acidic site and the medium Lewis acidic site. The Mn-loaded catalysts all showed a strong NH3 desorption peak at around 100˚C. The Mn-loaded catalysts all showed a strong NH3 desorption peak at around 100˚C. The desorption peak that appeared here belonged to the desorption of NH3 from the physisorbed state on the weak acidic sites and the dissociation of the Brønsted acidic sites, possibly accompanied by the binding to the weak Lewis acidic sites [11], Which indicated that the loading of the transition metal Mn increased the activity of the Brønsted acidic sites on the catalyst surface, number of acidic sites, resulting in increased activity in the low temperature section.
The NH3 desorption peak at 310˚C for the catalyst impregnated in 0.6 mol/L solution belonged to the strong adsorption of NH3 on the Brønsted acidic site and the moderate adsorption on the Lewis acidic site. The other three Mn-loaded catalysts all showed NH3 desorption peaks within 390˚C - 460˚C. Here the desorption peaks were caused by the combination of NH3 and moderate Lewis acidic sites [10], which indicated that the addition of the manganese nitrate solution resulted in a greater abundance of acidic sites on the catalyst surface. The peak area is generally considered to indicate the number of acidic sites. From Table 3, it can be obtained that the peak area gradually increased with the increase of loading, which indicated that the adsorption capacity of NH3 gradually increased, which was consistent with the activity test results. It is not difficult to find that the adsorption capacity of NH3 on the catalyst surface was
![]()
Table 3. Peak areas of NH3-TPD adsorption and desorption curves.
significantly enhanced when Mn elements were introduced into the synthetic (Ce,La)CO3F, and the adsorption capacity also increased with increasing loading, which indicated that the enhancement of the adsorption performance originated from chemical action [12].
Figure 5(b) showed the H2-TPR patterns of the (Ce,La)CO3F and Mn/(Ce,La)CO3F catalysts, From Figure 5(b), it can be seen that the (Ce,La)CO3F catalyst showed a reduction peak at 521˚C, where the reduction peak is attributed to the reduction of Ce4+ to Ce3+ [13], and with the increase in temperature, the reduction peak at 651˚C which was attributed to the reduction of the catalyst bulk phase CeO2 [14]. The oxide of La in (Ce,La)CO3F was La2O3, which also has a +2 valence state based on the arrangement of its outer electrons, and since La, Ce are neighbouring rare earth elements with relatively similar chemical properties, La may also have some redox ability. The catalysts loaded with Mn all showed reduction peaks at 450˚C - 490˚C. The reduction peaks here can be attributed to the process of MnO2/Mn2O3→Mn3O4→MnO conversion [15] [16], and due to the large number of MnOx species, the reaction process can be interconverted, which facilitates the redox reaction. The catalyst obtained by impregnation in a 0.4 mol/L solution, apart from the reduction of Mnn+ at 452˚C, the obvious change was the enhanced reduction of the bulk phase CeO2 at 661˚C and promoted the reaction. With increased loading, the position of the reduction peak of the bulk phase CeO2 shifted towards the low temperature section, and the H2 adsorption also increased significantly, Which indicated that strong electronic interactions between the MnOx species on the catalyst surface and CeO2 occurred, and the interactions promoted the reduction of Ce4+ on the surface. From Table 4, it can be seen the catalyst obtained by impregnation in 1.0 mol/L solution had the largest peak area compared to the other Mn-loaded catalysts, which indicated a higher redox capacity, the most active reduction of Ce4+ to Ce3+ under these conditions, the increase in the reduction potential of the active component, the formation of oxygen vacancies and more oxygen species, which facilitated the migration of oxygen and enhanced the activation reaction of the catalyst, and the catalyst has excellent redox ability.
3.5. Surface Element Valence Analysis
Figure 6(a) showed the Ce 3d spectrum. From Figure 6(a) it can be seen that
![]()
Table 4. Peak areas of H2-TPR adsorption and desorption curves.
the Ce 3d spectrum contains eight characteristic peaks. Of these, u (900.8 ev), u" (907.5 ev), u"' (916.5 ev), v (882.3 ev), v" (888.9 ev), v"' (898.4 ev) were attributed to Ce4+. u' (903.8 ev), v' (884.7 ev) had characteristic peaks that belonged to Ce3+ [17] [18]. It is obviously evident from Figure 6(a) that the Mn/(Ce,La)CO3F catalyst has a stronger peak at u', v' compared to the (Ce,La)CO3F catalyst, which implies an increased Ce3+ content on the catalyst surface. The higher Ce3+/(Ce4++Ce3+) ratio indicated that this catalyst exhibited an unsaturated chemical energy band and more oxygen vacancies, which would promote the adsorption of NH3 from the reactant [19] [20] [21], which is consistent with the results of NH3-TPD. The proportion of each type of element is calculated in Table 5, from which it can be obtained that by loading the transition metal Mn, the content of Ce3+ does increase compared to the original carrier, which indicated that the introduction of Mnn+ can cause Mn, Ce interaction, which makes part of Ce4+ to convert into Ce3+, promoting the redox ability of each other, which is beneficial to the SCR reaction. The elevated content of Ce3+ is beneficial to promote the transfer of oxygen on the catalyst surface and the regeneration of the active site in the redox cycle reaction.
Figure 6(b) showed the La 3d spectrum with electron binding energies of magnitude 854 - 855 ev, 850 - 851 ev, 837 - 838 ev and 833 - 834 ev [22]. The characteristic peaks of lanthanum metal are double peaks. When lanthanum metal forms a composite oxide La3d3/2 and La3d5/2 appear as companion peaks. The reason for the appearance of the companion peaks was the ionisation of electrons in the inner shell layers of La3d3/2 and La3d5/2, and the transfer of 2p valence electrons from the ligand oxygen with La to the 4f vacant orbital of La, causing the splitting of the La3d characteristic peak, which leads to the result of the vibronic companion peaks of La3d3/2 and La3d5/2 [23] [24], where the binding energy in the La 3d pattern of the synthetic (Ce,La)CO3F catalyst is lower than that of the standard characteristic peak and accompanying peaks. This indicated that there is no transfer of electrons between the metals La [25] and that a Ce-La-O solid solution may be formed here [26]. When loaded with the transition metal Mn, the electron binding energy of La3d5/2 was shifted more towards the lower end, probably due to the introduction of a third metal, which enhanced the inter-elemental interactions forming a new solid solution (Mn-Ce-La), which is consistent with the XRD results.
Figure 6(c) showed the O 1s spectrum of catalyst. The peak at 531.3 ev belonged to the surface adsorbed oxygen species, denoted as Oα, and the peak at 529.5 ev belonged to the lattice oxygen species, denoted as Oβ [27]. As can be seen from Figure 6(c), after loading with Mn, the lattice oxygen peak is slightly enhanced, but the adsorbed oxygen peak is weakened, but is similar to (Ce,La)CO3F. The chemisorbed oxygen (Oα) is the most active oxygen species
![]()
Table 5. Fitted data of XPS on the catalyst.
and plays a key role in the redox reaction [28]. From the calculations in Table 5, it can be seen that the (Ce,La)CO3F catalyst itself contains more surface adsorbed oxygen, and after loading with transition metals, the proportion of adsorbed oxygen decreases, which is probably because the addition of Mn does not contribute to the increase of chemisorbed oxygen (Oα). (Ce,La)CO3F and the catalyst after loading with the transition metal Mn, the binding energy of O 1s shifted slightly towards the lower compared to the standard binding energy. This suggested that O and the metal element interacted to form the Mn-O-Ce(La) species, which shifted to a lower binding energy due to its shorter bond length [29] [30].
Figure 6(d) showed the Mn 2p spectrum for the Mn/(Ce,La)CO3F catalyst. From Figure 6(d), the Mn 2p band can be obtained as two main peaks, Mn 2p1/2 (654.2 ev) and Mn 2p3/2 (641.1 ev). The Mn 2p of all catalysts can be further decomposed into three peaks, where the binding energy in the range of 641.0 - 641.3 ev belonged to Mn2+, 642.0 - 642.6 ev to Mn3+ and 644.0 - 644.7 ev to Mn4+ [31]. It is generally accepted that the catalytic capacity of MnOx is MnO2 > Mn3O4 > Mn3O4 [32] [33] [34] and that the catalyst surface produces a large amount of MnO2 to facilitate the SCR reaction [35]. The reason why Mn4+ plays more of a role in the NH3-SCR reaction than Mn3+ and Mn2+ is because its high redox ability can facilitate the conversion of NO to NO2 and enhance the catalytic activity of the low temperature section through the fast SCR reaction: NO + NO2 + 2NH3 = 3N2 + 2H2O, and therefore this reaction is also the main pathway for the low temperature reaction [36] [37].
4.In-Situ Infrared Analysis and Reaction Mechanism Study
In-Situ Infrared Spectroscopy of NH3 and NO+O2 Adsorption Stability on Catalyst Surfaces
In order to investigate the effect of catalyst activation by NH3 adsorption at optimum temperature conditions, in-situ IR spectroscopy was carried out on (Ce,La)CO3F and the catalysts produced after loading with Mn. From the activity test results, it was found that (Ce,La)CO3F reached the optimum activity at 200˚C, and Mn(1 mol/L)/(Ce,La)CO3F reached the optimum denitrification efficiency at 250˚C. This experiment investigated the variation of NH3 adsorption over time for both catalysts at the optimum denitrification temperature. Figure 7(a) showed the infrared spectra of NH3 with time for the synthetic (Ce,La)CO3F. From the figure, it can be seen that two distinct infrared absorption peaks appeared at 1590 cm−1 and 1301 cm−1 after 5 min of NH3 passage, the absorption peak at 1590 cm−1 was attributed to the NH3 species in the ligand state on the Lewis acidic site [38], and the absorption peak at 1301 cm−1 is attributed to the dehydrogenation product eNH2 produced by the dehydrogenation reaction of the activated NH3 species [39]. With increased passage time of NH3, the NH3 species adsorbed at the Lewis acidic site at 1590 cm−1 disappeared at 10 min, which indicated that it was very unstable with time. At 20 - 30 min pass,
![]()
Figure 7. In-situ infrared spectra of NH3 adsorption of the catalyst at the optimal denitration temperature over time. (a) (Ce,La)CO3F. (b) Mn(1 mol/L)/(Ce,La)CO3F.
three IR absorption peaks appeared on the catalyst surface at 1540 cm−1, 1442 cm−1 and 1401 cm−1. The absorption peak at 1540 cm−1 was attributed to the V3 splitting pattern of bidentate nitrate, and the appearance of this peak was attributed to the oxidative adsorption of NH3 by the oxygen species on the catalyst surface [40]. The absorption peaks at 1442 cm−1 and 1401 cm−1 belonged to
species on the Brønsted acidic sites [41] [42], which indicated that the synthetic (Ce,La)CO3F catalyst was relatively rich in acidic sites on the surface, but that these species were extremely unstable and decomposed and disappeared as the NH3 influx time changed. Finally, the stability of the NH3/
species formed was observed by purging with N2 for 30 min. It was found that after the N2 purge, most of the NH3/
species formed on the catalyst surface disappeared, with only the dehydrogenation product eNH2 produced by the NH3 species at 1301 cm−1 remaining stable, and thus participated in the NH3-SCR reaction. Notably, after N2 purging, the catalyst showed a new IR absorption peak at 1518 cm−1, where the peak was due to the dehydrogenation of -NH2 species from NH3 species adsorbed on the Brønsted acidic site [40].
Figure 7(b) showed the infrared spectrum of NH3 adsorption with time for the Mn/(Ce,La)CO3F catalyst at 250˚C. From Figure 7(b), it can be seen that when NH3 was introduced for 5 min, infrared absorption peaks appeared on the catalyst surface at 1610 cm−1, 1409 cm−1 and 1224 cm−1, with the absorption peak at 1610 cm−1 attributed to the NH3 species in the Lewis acidic site [41], and this species disappeared quickly with increasing time, which indicated its unstable adsorption. The absorption peaks at 1409 cm−1 and 1224 cm−1 are attributed to asymmetric bending vibrations occurring in the
species at the Brønsted acidic site [43] [44] and the NH3 species at the Lewis acidic site, respectively. The infrared absorption peak at 1653 cm−1 on the catalyst surface after 10 min of passage belongs to the
species at the Brønsted acidic site [45] [46]. This indicated that Mn/(Ce,La)CO3F was dominated by Brønsted acidic sites on the catalyst surface at 250˚C, accompanied by a small amount of Lewis acidic sites to promote the reaction. This suggested that the use of manganese nitrate solution as a precursor could provide more Brønsted acidic sites for the catalyst. It has been reported in the literature that Brønsted acidic sites have a facilitative effect on the NH3-SCR reaction [47], and after loading, the NH3/
species produced by NH3 adsorption were more stable compared to (Ce,La)CO3F. The species were present in a very stable state on the catalyst surface, both with increasing NH3 influx time and N2 purging, thus facilitating the NH3-SCR reaction. In addition, the intensity of the absorption peaks of the species adsorbed on the Lewis and Brønsted acidic sites increased slightly, probably because the nitric acid caused some cracks on the catalyst surface, resulting in more oxygen vacancies on the catalyst surface, which facilitated the adsorption of Ce4+ on the acidic sites.
Compared to both catalysts, the NH3/
species formed on the surface of the Mn/(Ce,La)CO3F catalyst are more stable and have more Brønsted acidic sites as well as oxygen vacancies, allowing more Ce4+ in the carrier to participate in the reaction in combination with the NH3/
species.
To further investigate the effect of catalysts on NO adsorption and activation performance, the in-situ infrared spectra of NO+O2 adsorption with time for two different catalysts at the optimum denitrification temperature conditions were also investigated, as shown in Figure 8. Figure 8(a) showed the infrared spectrum of NO+O2 adsorption with time for the synthetic (Ce,La)CO3F catalyst at 200˚C. It can be found that (Ce,La)CO3F showed two IR absorption peaks on
![]()
Figure 8. In-situ infrared spectra of NO+O2 adsorption of the catalyst at the optimal denitration temperature over time. (a) (Ce,La)CO3F. (b) Mn(1 mol/L)/(Ce,La)CO3F.
the catalyst surface at 1510 cm−1 and 1338 cm−1 at 5 min of NO+O2 pass, the absorption peak at 1510 cm−1 belonged to a monodentate nitrate species [48], and the absorption peak at 1338 cm−1 is attributed to a bidentate nitrate species [49], with the NO+O2 passage time increased, the absorption peak of the bidentate nitrate species was found to disappear gradually, which indicated that its adsorption on the catalyst surface was less stable and prone to decomposition. The absorption peak of the monodentate nitrate species at 1510 cm−1 was shifted to 1490 cm−1 at 10 min of NO+O2, where the nitrate species was still monodentate, probably due to the large amount of monodentate nitrate species produced during the reaction. At 10 min IR absorption peaks appeared at 1634 cm−1 and 1239 cm−1 on the catalyst surface, the absorption peak at 1634 cm−1 belonged to the bidentate nitrate species and weak adsorption could be found. The peak at 1239 cm−1 belonged to the bridging nitrate species [49] [50]. With increasing NO+O2 influx time and final purging with N2, the monodentate nitrate, bidentate nitrate and bridging nitrate species were all present on the catalyst surface in a very stable state.
Figure 8(b) showed the in situ IR spectra of NO+O2 adsorption over time for the Mn/(Ce,La)CO3F catalyst at 250˚C. From Figure 8(b), it can be seen that there is no obvious large trend of IR absorption peaks on the catalyst surface when NO+O2 is introduced for 5 min. With increasing NO+O2 pass time, strong IR absorption peaks appeared on the catalyst surface at 1436 cm−1 and 1204 cm−1 at 10 min. The absorption peaks at 1436 cm−1 belonged to monodentate nitrate species, and the absorption peaks at 1204 cm−1 were attributed to nitrate species bound at the Mn-O-Ce site [51] [52], and similarly at 10 min, the absorption peak at 1653 cm−1 belonged to the bidentate nitrate species and the bidentate nitrate species was present in a very stable state as the NO+O2 pass time increased. At 20 min, the IR absorption peaks at 1511 cm−1, 1455 cm−1 and 1292 cm−1 on the catalyst surface were all attributed to the monodentate species [49] [53], and the monodentate species was very stable after N2 purging. It is noteworthy that the number of monodentate nitrate species on the catalyst surface increased with Mn loading compared to the (Ce,La)CO3F catalyst, with a new nitrate species (bridged nitrate) appearing at 1204 cm−1 at 10 min, so it can be judged that these nitrate species bonded to the Mn3+ provided by the loaded Mn to form intermediate products that participated in the SCR reaction. In addition, the absorption peak intensities of the monodentate nitrate species were also greatly increased by the loading of Mn.
In this experiment, the process of NH3 adsorption and activation on the catalyst surface under different temperature conditions was investigated by in situ infrared spectroscopy for both catalysts, and the forms of NH3 species present on the catalyst surface under different temperature conditions were studied. Figure 9(a) showed the spectrum of the change in thermal stability of the NH3 adsorbed species of (Ce,La)CO3F in the interval of 50˚C - 400˚C. Firstly, it can be observed that throughout the temperature interval, two distinct infrared absorption peaks appear on the catalyst surface at 1624 cm−1 and 1317 cm−1. The absorption peak
![]()
Figure 9. In-situ infrared spectra of NH3 adsorbed species on the catalyst surface under different temperature conditions. (a) (Ce,La)CO3F. (b) Mn(1 mol/L)/(Ce,La)CO3F.
at 1624 cm−1 belonged to NH2, an intermediate product of the dehydrogenation reaction of the NH3 species at the Lewis acidic site [43]. The absorption peak at 1317 cm−1 belonged to the
species at the Brønsted acidic site [46], so it can be assumed that the Brønsted/Lewis acidic site is involved in the reaction throughout the temperature interval. It is noteworthy that in the range of 150˚C - 200˚C, an infrared absorption peak appeared on the catalyst surface at 1441 cm−1, where the absorption peak belonged to the
species on the Brønsted acidic site [39], which indicated that more acidic sites were involved in the reaction in the range of 150˚C - 200˚C, with the Brønsted acidic site being the main acidic site, which favoured the catalytic reaction, which is consistent with the activity test results. As the temperature increased, the absorption peak at 1441 cm−1 was shifted to 1492 cm−1 and the intensity of the absorption peak was weakened, resulting in a decrease in denitrification efficiency. Overall, it appears that the (Ce,La)CO3F catalyst has a weak peak intensity throughout the reaction temperature range, which indicated that this catalyst has a poor adsorption capacity for NH3, which is the reason for the low denitrification efficiency.
Figure 9(b) showed the variation of the thermal stability of the Mn/(Ce,La)CO3F catalyst for NH3 adsorbed species in the interval 50˚C - 400˚C. From Figure 9(b), it can be observed that a strong IR absorption peak at 1512 cm−1 appears on the catalyst surface in the temperature range of 50˚C - 300˚C. The absorption peak here is attributed to the amide (−NH2) species produced by the dehydrogenation reaction of
species adsorbed on the Brønsted acidic site, in the temperature interval of 50˚C - 300˚C, the Brø nsted acidic sites play a major role, which also indicated that the manganese nitrate solution could provide more Brønsted acidic sites for the catalyst. It can also be observed that within the low temperature section (50˚C - 150˚C), infrared absorption peaks appear on the catalyst surface at 1669 cm−1 and 1358 cm−1, with the absorption peak at 1669 cm−1 belonged to the
species on the Brønsted acidic site [44] and the absorption peak at 1358 cm−1 belonged to the deformation vibration of the N-H bond on the Brønsted acidic site. The Lewis acidic site was not detected throughout the low temperature section, which indicated that the Brønsted acidic site occupied the main active site in the low temperature section. With increasing temperature, only the amide (-NH2) species at 1512 cm−1 was stable in the range of 200˚C - 300˚C. All other absorption peaks belonged to the Brønsted acidic site underwent pyrolysis and desorption, but were accompanied by the appearance of some new absorption peaks. The absorption peak at 1657 cm−1 belonged to the intermediate product −NH2 species after dehydrogenation of NH3, and the absorption peak at 1305 cm−1 belonged to the eNH2 species generated by the dehydrogenation reaction of the activated NH3 species [39]. According to the denitrification activity test results, it can be obtained that the denitrification rate decreases to a certain extent at 200˚C, and then there is a substantial recovery at 250˚C. From Figure 9(b), it can be seen that the
species at 1358 cm−1 and 1669 cm−1 decompose when the reaction temperature reaches 200˚C, and only the amide (-NH2) species at 1512 cm−1 is involved in the reaction on the catalyst surface, so causing such a phenomenon to occur.
The process of NO+O2 species adsorption and activation on the catalyst surface under different temperature conditions was investigated by in situ infrared spectroscopy to discuss the forms and changes of NOx species present on the catalyst surface under different temperature conditions. Figure 10(a) showed the infrared variation spectrum of thermal stability of NO+O2 adsorbed species on the (Ce,La)CO3F catalyst in the temperature interval of 50˚C - 400˚C. Firstly, it can be observed that only one IR absorption peak appears on the catalyst surface at 1438 cm−1 in the temperature interval 50˚C - 100˚C. The absorption peak here can be attributed to the monodentate nitrate species [51], but as the temperature increases, the monodentate species decomposes, so it only occupies the
![]()
Figure 10. In-situ infrared spectra of NO+O2 adsorbed species on the catalyst surface under different temperature conditions. (a) (Ce,La)CO3F. (b) Mn(1 mol/L)/(Ce,La)CO3F.
active site in the low temperature section. In the temperature interval of 150˚C - 250˚C, IR absorption peaks appeared on the catalyst surface at 1288 cm−1, 1386 cm−1 and 1602 cm−1. The absorption peaks at 1288 cm−1 and 1386 cm−1 both belonged to monodentate nitrate species [43] [49], and the IR absorption peak at 1602 cm−1 belonged to the Ce-O-Ce site on the NO2 species [38]. It is clear that only some of the monodentate nitrate species are relatively more stable in this temperature interval, occupying the active sites in the middle and high temperature bands. As the temperature increased, the NO2 species disappeared and a new monodentate nitrate species appeared at 1522 cm−1. Throughout the temperature interval, the nitrate species produced by adsorbed NO were dominated by monodentate nitrate, but the adsorption and its instability and susceptibility to decomposition by temperature contributed to the poor denitrification activity.
Figure 10(b) showed the IR variation spectrum of the thermal stability of the NO+O2 adsorbed species of the Mn/(Ce,La)CO3F catalyst in the interval of 50˚C - 400˚C. As can be seen from Figure 10(b), strong IR absorption peaks appear on the catalyst surface at 1208 cm−1, 1482 cm−1 and 1610 cm−1 in the temperature interval of 50˚C - 250˚C. The absorption peaks at 1208 cm−1 and 1482 cm−1 belonged to the monodentate nitrate species formed on the Mn-O-Ce site [51]. The absorption peak at 1610 cm−1 belonged to NO2 species adsorbed on the catalyst surface [45]. It has been reported in the literature that NO2 species are important intermediates in the fast SCR reaction under low temperature conditions and also an important factor in improving the catalytic performance of the catalyst [54] [55] [56]. As the temperature continues to rise, the NO2 species disappears, which indicates that it is less stable and prone to decomposition, while the monodentate nitrate species is relatively more stable. The absorption peak at 1511 cm−1 is a monodentate species formed by the shift of the absorption peak at 1482 cm−1. The shift and increase in peak intensity is due to the gradual formation of more monodentate species on the catalyst surface, so that until 250˚C the monodentate species occupies the main active site in the reaction. As the temperature continues to increase, the peak of the monodentate nitrate species gradually disappears and a peak belonged to the bidentate nitrate species appears at 1341 cm−1. The high temperature inactivation may be due to the fact that Mn3+ and Ce3+ only bond with the monodentate nitrate species to form O-Mn3+-O-NO and O-Ce3+-O-NO intermediates to participate in the reaction, and not with the other nitrate species. Compared to the synthetic (Ce,La)CO3F catalyst, the loading of Mn resulted in the formation of a greater number of monodentate nitrates and a very significant increase in the peak intensity of the absorption peaks, which in turn bound transition metal ions to participate in the reaction. There is a reduction in the number of nitrate species on the catalyst surface, but an increase in the absorption peak intensity of the monodentate species, which occupies the active site throughout the denitration temperature band.
To further investigate the NH3-SCR reaction mechanism of the catalyst, in-situ IR spectroscopy of the catalyst was carried out under different reaction conditions at the optimum denitrification temperature. Figure 11(a) showed the
![]()
Figure 11. In-situ infrared spectra of the reaction of NH3 pre-adsorbed species on the catalyst surface under the optimal reaction temperature.
insitu IR spectra of the reaction of NO+O2 on the surface of the (Ce,La)CO3F catalyst with preadsorbed NH3 species at 200˚C. The NH3 gas was first introduced into the IR reaction cell containing the catalyst for 60 min, before which N2 was used as a supplementary gas to pretreat the catalyst at 200˚C for 30 min, then the NH3 gas was stopped and purged with N2 for 10 min. The changes in the IR spectrum of the catalyst surface were observed at 200˚C. After first adsorption of NH3 for 1 h, it can be found that more stable NH3 adsorption species appear on the catalyst surface at 1638 cm−1, 1457 cm−1, 1310 cm−1 and 1060 cm−1. The IR absorption peaks at 1638 cm−1 and 1060 cm−1 are attributed to NH3 species on the Lewis acidic site [57], and the IR absorption peaks at 1457 cm−1 and 1310 cm−1 are attributed to
species on the Brønsted acidic site. NH3 was switched off and NO+O2 gas was then introduced into the reaction cell. When NO+O2 was introduced for 2 min, the
species on the Brønsted acidic sites at 1457 cm−1 and 1310 cm−1 and the NH3 species belonging to the Lewis acidic site at 1060 cm−1 disappeared rapidly, and only the absorption peak at 1638 cm−1 was present, which indicated that the NH3/
species could react rapidly with NO here. The E-R reaction mechanism exists on the surface of the (Ce,La)CO3F catalyst and the main reacting species are NH3/
species and NO species. With increasing NO+O2 influx time, the infrared absorption peaks of NO adsorbed species appear at 1489 cm−1 and 1356 cm−1 on the catalyst surface at 5 min, both of which are monodentate nitrate species. At 10 min the absorption peaks of the monodentate nitrate species weakened, and according to the IR profiles of the (Ce,La)CO3F catalysts for NO+O2 adsorption over time, the monodentate nitrate species were very stable over time, so the weakening of the monodentate nitrate here was probably due to the reaction with the incompletely reacted NH3/
species, which suggested that the L-H mechanism was also present here, with the main reacting species were NH3/
species and O-Ce3+-O-NO species. With increasing time, many new spectral bands of nitrate species appeared on the catalyst surface. The IR absorption peaks at 1363 cm−1 and 1350 cm−1 belong to monodentate nitrate species adsorbed on the Ce-O-Ce sites on the catalyst surface, and the IR absorption peaks at 1588 cm−1 and 1246 cm−1 belonged to bridged nitrate species [58]. the peak at 1057 cm−1 belonged to the linked secondary nitrate species [−(N2O2)2−] [59] [60].
Figure 11(b) showed the in situ IR spectra of the reaction of NO+O2 on the surface of the Mn/(Ce,La)CO3F catalyst with preadsorbed NH3 species at 250˚C. When NH3 was introduced into the reaction cell system for 60 min at 250˚C, IR absorption peaks could be observed on the catalyst surface at 1672 cm−1, 1102 cm−1, 1400 cm−1, 1286 cm−1 and 1048 cm−1, with the absorption peaks at 1672 cm−1 and 1048 cm−1 belonged to the NH3 species at the Lewis acid site. The absorption peaks at 1102 cm−1, 1400 cm−1 belonged to the
species at the Brønsted acid position and the absorption peak at 1286 cm−1 belonged to the e-NH2 species generated by
dehydrogenation. With the passage of NO+O2, the NH3 adsorbed species all disappeared at 2 min, which indicated that NH3/
/e-NH2 species can react with NO very quickly, so this catalyst surface follows the E-R mechanism. As the NO+O2 pass time increased, nitrate species appeared on the Mn/(Ce,La)CO3F catalyst surface at 5 min pass, with a bidentate nitrate species (1646 cm−1), a monodentate nitrate species on the Mn-O-Ce site or the Ce-O-Ce site (1220 cm−1), even a secondary nitrate [−(N2O2)2−] (1052 cm−1), and monodentate nitrate species (1500 cm−1, 1425 cm−1), but the disappearance of the absorption peak at 1500 cm−1 at 10 min was not due to a reaction with the NH3 adsorbed species, but to the production of a large number of monodentate nitrate species causing the absorption peak at 1500 cm−1 to be shifted to 1425 cm−1. This suggested that the Mn/(Ce,La)CO3F catalyst followed the E-R mechanism at 250˚C with NH3/
/e-NH2 and NO as the reacting species.
Figure 12 showed the in-situ IR spectra of the reaction of NH3 with pre-adsorbed NO+O2 species for both catalysts at optimum denitration temperature conditions. Figure 12(a) showed the transient in-situ IR spectrum of the (Ce,La)CO3F catalyst at 200˚C temperature conditions. When NO+O2 was introduced into the reaction cell system for 60 min, distinct IR absorption peaks appeared on the catalyst surface at 1618 cm−1, 1489 cm−1, 1340 cm−1 and 1252 cm−1, with the absorption peaks at 1618 cm−1 attributed to NO2 species (nitro or NO2 molecules) adsorbed on the Ce-O-Ce sites. Monodentate nitrate species (1489 cm−1), bidentate nitrate species (1340 cm−1), and bridged nitrate species (1340 cm−1) were also present, all of which were already present in a stable state on the catalyst surface. Turning off NO+O2, NH3 gas was then passed into the reaction cell. With the introduction of NH3, it was observed that at 2 min the nitrate species generated on the surface of the (Ce,La)CO3F catalyst disappeared and only the bridged nitrate species were present, but at 5 min they also participated in the reaction, which indicated that the NO2 species, monodentate nitrate
![]()
Figure 12. In-situ infrared spectra of the reaction of NO+O2 pre-adsorbed species on the catalyst surface under the optimal reaction temperature. (a) (Ce,La)CO3F. (b) Mn(1 mol/L)/(Ce,La)CO3F.
and bridged nitrate species were involved in the reaction with the NH3 adsorbed species. With increased NH3 passage time,
species belonging to adsorbed on Brønsted acidic sites (1404 cm−1, 1369 cm−1, 1368 cm−1, 1391 cm−1) and NH3 species belonging to Lewis acidic sites (1588 cm−1, 1307 cm−1, 1267 cm−1) and amide species -NH2 (1507 cm−1, 1502 cm−1, 1511 cm−1) appeared on the catalyst surface, and the amide species -NH2 (1507 cm−1, 1502 cm−1, 1511 cm−1), which suggested that an L-H mechanism also exists on the surface of the (Ce,La)CO3F catalyst. The NH3 species on the Lewis acidic site are less stable and prone to decomposition as can be seen from the graph. The absorption peaks of the monodentate nitrate species are the most intense among the adsorbed species of NO, so the main reactive species for the L-H mechanism are the
species on the Brønsted acidic site and the monodentate nitrate species bound to Ce3+.
Figure 12(b) showed the in situ IR spectra of the reaction of NH3 on the surface of the Mn/(Ce,La)CO3F catalyst with pre-adsorbed NO+O2 species at 250˚C. When NO+O2 was passed into the reaction cell system for 60 min at 250˚C, infrared absorption peaks could be observed on the catalyst surface at 1690 cm−1, 1508 cm−1, 1441 cm−1 and 1299 cm−1, which belonged to the monodentate nitrate species (1508 cm−1, 1299 cm−1), the bidentate nitrate species (1690 cm−1) and the trans -N2O4 cis -N2O2 species (1441 cm−1), which decomposes NO due to the reduction of NO2 by Ce3+. When NH3 was introduced, the absorption peaks of monodentate as well as bidentate nitrate species disappeared at 2 min, here in reaction with NH3 adsorbed species, so that an L-H mechanism also existed on the Mn/(Ce,La)CO3F catalyst surface. With the passage of NH3,
species belonged to the Brønsted acidic site (1402 cm−1, 1491 cm−1, 1525 cm−1, 1508 cm−1, 1540 cm−1) and NH3 species belonging to the Lewis acidic site (1368 cm−1, 1263 cm−1) appear on the catalyst surface. It can be clearly seen that the Brønsted acidic sites on the catalyst surface are the most abundant and the NO adsorption species have the highest intensity of the monodentate nitrate absorption peak, so the main reactive species for the L-H mechanism are the
species and the O-Ce3+-O-NO and O-Mn3+-O-NO species.
The IR spectrum of the (Ce,La)CO3F catalyst reveals E-R mechanism and L-H mechanism at an optimum denitrification temperature of 200˚C. The reactive species in the L-H mechanism are
species adsorbed on the Brønsted acidic site and monodentate nitrate species bonded to Ce3+.
The reaction process of the L-H mechanism on the surface of the (Ce,La)CO3F catalyst is as follows.
(
NO adsorption sites on catalyst surfaces) (1)
(monodentate nitrate) (2)
(Brønsted) (3)
(Brønsted) (4)
(5)
(6)
(7)
Mn/(Ce,La)CO3F catalysts were subjected to E-R and L-H mechanisms at 250˚C. After loading with the transition metal Mn, the number of Brønsted acidic sites on the catalyst surface was increased, enhancing the ability to adsorb NH3 on the catalyst surface. The number of nitrate species was relatively reduced, but the number and peak strength of monodentate nitrate increased, so that the main reacting species for the L-H mechanism of the Mn/(Ce,La)CO3F catalyst were monodentate nitrate species bonded to the metal ions Mn+ (Ce4+, Mn4+) and
species adsorbed on the Brønsted acidic sites. The reactions proceeded as follows. The L-H mechanism was shown in Figure 13.
(
Newly introduced Mn provides NO adsorption site) (8)
(monodentate nitrate) (9)
(Brønsted) (10)
(Brønsted) (11)
(12)
(13)
(14)
The E-R reaction mechanism is also present on the surface of the (Ce,La)CO3F catalyst at 200˚C. The reacting species are NO and
species at the Brønsted acidic site on the catalyst surface, and the E-R mechanism reaction
![]()
Figure 13. Schematic diagram of the L-H mechanism for the synthesis of (Ce,La)CO3F and Mn-loaded catalysts.
![]()
Figure 14. Schematic diagram of the E-R mechanism for the synthesis of (Ce,La)CO3F and Mn-loaded catalysts.
proceeds as follows.
(Brønstedacidic sites) (15)
(Brønsted) (16)
(17)
(18)
(19)
The Mn/(Ce,La)CO3F catalyst also has an E-R mechanism at 250˚C. The reacting species are mainly NO and
species adsorbed on the acidic sites of Brønsted. The E-R mechanism reaction process of the Mn/(Ce,La)CO3F catalyst is as follows, where M(Mn4+, Ce4+). The E-R mechanism was shown in Figure 14.
(Brønsted) (20)
(Brønsted) (21)
(22)
(23)
5. Conclusion
In accordance with the cerium-lanthanum ratio of fluorocerium ores in the mineralogy of the Baiyun Ebo process, pure substances such as Ce(NO3)3·6H2O, La(NO3)3·6H2O were used to synthesize (Ce,La)CO3F grains to simulate bastnaesite minerals by hydrothermal method, and used as NH3-SCR denitrification catalysts. After being roasted at a series of different temperatures, the catalyst surface produced a well-crystallised Ce7O12 species as the active component for denitrification. The activity results showed that the synthetic (Ce,La)CO3F was roasted at 500˚C, and the NOx conversion was 27% at 200˚C. The NH3-SCR catalytic activity of the synthesised (Ce,La)CO3F was improved by loaded transition metal Mn. The best catalyst was found to be produced by impregnating (Ce,La)CO3F with 1 mol/L manganese nitrate solution, with a NOx conversion of 80% at 250˚C. The physicochemical properties were analysed using XRD, BET, H2-TPR, NH3-TPD and XPS. The loading of Mn resulted in the appearance of numerous well-dispersed MnOx species on the catalyst surface, the dispersion of Ce7O12 species was also greatly enhanced, and the reduction in grain size indicated that Mnn+ entered into the (Ce,La)CO3F lattice causing lattice shrinkage. The number of acidic sites on the catalyst surface and the redox capacity were enhanced. The amount of Ce3+ in the catalyst was also enhanced by the introduction of Mnn+, but the proportion of adsorbed oxygen decreased, which indicated that the introduction of Mnn+ was detrimental to the increase in the proportion of adsorbed oxygen. The reaction mechanisms of the (Ce,La)CO3F and Mn/(Ce,La)CO3F catalysts were investigated byin-situ Fourier transform infrared spectroscopy (FTIR), to provide theoretical guidance for the specific reaction pathways of bastnaesite in the NH3-SCR reaction. The results showed that catalysts followed both the E-R and L-H mechanisms throughout the reaction process. When loaded with Mn, the main reactive species in the L-H mechanism were the
(ad) species on the Brønsted acidic site and the O-Ce3+-O-NO, O-Mn3+-O-NO species. The main reactive species for the E-R mechanism were NH3/
(ad) species on the Brønsted/Lewis acidic sites and NO. The
(ad) species on the Brønsted acidic sites act as the main reactive NH3(g) adsorbing species, bonded to the Ce4+ in the carrier (Ce,La)CO3F to participate in the acid cycle reaction. The introduction of Mnn+ increases the number of Brønsted acidic sites on the catalyst surface, and acts as an adsorption site for NO, to react with NO to generate more monodentate nitrate species, to participate in the redox cycle reactions. The above results indicated that Mnn+ and (Ce,La)CO3F have a good mutual promotion effect, which makes the loaded catalyst have excellent performance, which provides a theoretical basis for the high value utilization of bastnaesite.
Acknowledgements
This study was financially supported by Natural Science Foundation of Inner Mongolia (Grant No. 2019ZD13, 2020BS05030), National Natural Science Foundation of China (Grant No. 51866013). Thanks for Start-up Funds for Talent Introduction and Scientific Research of Institutions in Inner Mongolia Autonomous Region.