1. Introduction
The oral cavity is home to one of the most complex bacterial ecosystems in the body. Several hundred species of microorganisms coexist in the oral environment: bacteria, yeasts, protozoa and viruses. This natural cavity, along with the colon, is the most septic part of the human body.
Many authors have tried to quantify it: a milligram of plaque contains approximately 100 million bacteria; 1 milliliter of saliva contains an average number of 750 million bacteria (including 100 million bacteria that can be cultivated on culture medium) [1].
During orthodontic treatment, we use a fixed appliance constituted of several components and biomaterials including stainless steel. In the oral cavity, these metallic biomaterials are exposed to many factors such as saliva, bacterial microflora, food, temperature fluctuations, and mechanical forces. Leading to ecological changes in the oral environment and increase of Streptococcus mutans count in the saliva and dental plaque [2].
The formation of biofilm in the oral cavity is a gradated process consisting of four distinct stages:
1) Acquired pellicle formation;
2) Primary (early) colonization;
3) Secondary colonization/co-aggregation;
4) Mature biofilm establishment
Plaque accumulation, which can lead to WSL (White spots Lesion) formation and gingival inflammation, represent a significant challenge to excellence in clinical orthodontics. White spots and enamel demineralization around orthodontic brackets are among the most important complications resulting from orthodontic treatments [2] [3].
The process of oral biofilm creation proceeds at the dental and biomaterial interface, where saliva plays an important role. Glycoproteins and phosphoproteins present in saliva, such as mucins and proline-rich proteins adhere to the bacteria-free surfaces of teeth, oral mucosa, and biomaterials through ionic, van der Waals, and hydrophobic interactions [4] [5] [6] [7].
Adsorption of proteins changes electrochemically the tooth and biomaterial surfaces, which mediates interactions with the microbe-rich oral environment. In effect, microorganisms interact directly with built-in film forming molecules which have an influence on the further colonization of adsorbed microbes [8] [9].
1.1. Biofilm Formation
After adsorption of microorganisms by the surface of the pellicle, a further and faster, adhesion of microorganisms together with glycoproteins and phosphoproteins is observed. With time, so-called dental plaque and denture plaque are formed [10].
An important role in the process of biofilm growth is performed by saliva, which is a transporter of nutrients for persistent microorganisms in root canals. Also, saliva is a carrier of antimicrobial compounds: lysozyme, lactoferrin, sialo peroxidase, histatin, Statherian, and bacteriocin [10].
Therefore, it is the main source of substances necessary for producing the extracellular matrix (EPS), that, together with bacteria, forms a biofilm. This matrix is like a scaffold for bacteria, enabling it development and providing protection against the external environment [11] [12].
Constant saliva flow makes colonization of the oral cavity difficult for microorganisms and, to some extent, ensures control of biofilm growth [13]. Mucins and other glycoproteins contribute to this process through aggregating bacteria into larger complexes and attaching them to the mucosal surface, blocking the adhesion of other bacteria. This process is part of a protective mechanism against pathogenic organisms [14].
The process of biofilm formation in the oral cavity can be described as follows [15]:
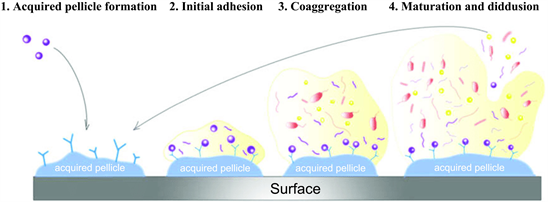
The human saliva produces the main source of nutriments for microorganism adhesion and it allows the coating of hard or soft surfaces by a thin (5 - 10 µm thickness), heterogeneous and acellular pellicle, named acquired pellicle or conditioning film oralis. Germs are weakly and reversibly linked to the acquired pellicle by adhesins, although they may remain and proliferate, starting the phenomena of microbial co-aggregation, Streptococcus species represent 60% - 80% of all primary colonizers. Such co-aggregation is mediated by metabolic and genetic exchange known as quorum sensing [16]. The secondary adhesion occurs within 3 to 5 days after the beginning of the acquired pellicle deposition. In this process, the microorganisms start to grow and co-aggregate. The biofilm maturation is achieved within 2 to 3 weeks [17].
The biofilm formation on materials for dentistry also depends on oxygen, nutriments and PH [18] [19].
1.2. Quorum Sensing
Some of the unique functions of biofilms are dependent on the ability of the bacteria and micro-colonies within the biofilm to communicate with one another. Quorum sensing or cell density mediated gene expression in the bacteria involves regulation of expression of specific genes through the accumulation of signaling compounds that generate intercellular communication. Quorum-sensing signaling represents a signaling pathway that is activated as a response to cell density. Such systems are found in both Gram-positive and Gram-negative micro-organisms. The stimuli of quorum-sensing systems are signal molecules, called autoinducers [20] [21].
The aim of this work was to study in vitro the behavior of certain bacteria of the oral flora in contact with a dental surface and stainless steel to:
- Observe the ability of these germs to adhere to dental surfaces and to stainless steel surfaces.
- Quantitatively assess the adhesion and proliferation potential of these germs in contact with dental surfaces and titanium.
- Compare their behavior on contact with these 2 surfaces.
2. Materials and Methods
- Biomaterials
Cylinders in Stainless steel (AISI 316L), commonly called stainless steel or stainless steel, is an alloy of steel (comprising less than 1.2% carbon) with more than 10.5% chromium, the property of which is to be little susceptible to corrosion and not degrading to rust. These cylinders were machined using a disc placed on a mandrel carried by a handpiece, in different samples of 5 mm in length and 3 mm in width and 2 mm in thickness.
Freshly extracted permanent natural teeth were cut lengthwise into different samples 5 mm long and 3 mm wide and 2 mm thick, consisting mainly of enamel, the outer layer of the crown of the tooth.
The dental fragments and the titanium fragments were disinfected at (Hexanios G + R, Laboratoire Anios) for 15 minutes and then sterilized in a wet autoclave (Tau Clave 3000, Vacuum) at 120˚C for 30 min.
- Bacterial strains
The reference bacterial strain that we used in our study is Staphylococcus aureus Méti S ATCC 29213.
Staphylococcus aureus is the most pathogenic species of the genus Staphylococcus, both in humans and animals [17]. This staphylococcus is non-motile and occurs in shells of 0.5 to 1.5 pm in diameter associated in pairs or in clusters.
The strain was stored in the form of an aliquot in a medium composed of heart-brain broth (BHI) added with 10% glycerol and frozen at −20˚C.
- Reactivation of germs
The reference strain used is cultured, by placing it in a liquid enrichment medium (BHI) and incubated in the oven at 37˚C for 2 to 4 hours. Then a drop of this incubated broth was inoculated with a sterile loop, in the media suitable for the culture of this strain:
- Chocolate agar composed of Columbia agar with cooked blood added with poly vitamin supplements allowing the growth of all bacterial strains and especially the deficient bacteria.
- Chapman agar (medium hypersalé at 7% Nacl) selective for staphylococci.
The inoculated dishes are incubated in the oven at 37˚C for 18 to 24 hours under 5% CO2.
- Preparation of the media necessary for bacterial adhesion to biomaterials
We used two pairs of boxes from each of the following media: MHS (Mueller Hinton = Mueller − Hinton + Fresh Blood), MH (Mueller Hinton) and chocolate agar:
- The first pair of boxes contains two dental fragments embedded in the agar so that the amelar surface is on the same plane as the agar surface, we chose the amelar surface because it is the first surface in contact with the bacteria in the oral cavity.
- The second pair of boxes each contains 2 stainless steel fragments embedded in the agar presenting an accessible surface and on the same plane as the agar.
- Bacterial strain confirmation tests
- Confirmation by sowing on Chapmen medium:
Chapman agar is the selective medium for halophilic bacteria and more particularly fermenting red mannitol. It is a semi-synthetic medium. It is used for the isolation of Staphylococcus.
For the preparation of a liter of medium we need:
Peptone: 10.0 g
Beef extract: 1.0 g
Sodium chloride: 75.0 g
Mannitol: 10.0 g
Phenol red: 0.025 g
Agar-Agar: 15.0 g
Distilled water: 1 l
111 g/l of medium: Classic autoclaving at 120˚C for 20 minutes
This environment is characterized by:
- An ordinary nutritional base.
- A high NaCl content which allows the selection of halophilic bacteria (such as Staphylococcus) and inhibits the vast majority of other bacteria.
- A differentiation criterion: the fermentation of mannitol revealed thanks to the change in the colored pH indicator: phenol red which allows orientation towards certain species (such as the Staphylococcus aureus species).
In a Chapmen tube, a few colonies of staphylococcus aureus ATCC 29213 were inoculated using a plastic handle on the slope of the agar. The observation was made after 24 hours of incubation at 37˚C.
- Confirmation by biochemical catalase test
This test is the basis for the identification of Gram + bacteria. On a clean slide, a few colonies were deposited by a loop with a few drops of the catalase reagent in order to observe the behavior of the bacteria.
- Confirmation by Gram staining
Gram stain (developed by Christian Gram) is a basic stain in bacteriology. It is a “double coloring”, which makes it possible to differentiate bacteria:
- According to their shape
- According to their affinity for dyes.
+ Preparation of the smear
A new blade was used. On the perfectly cold slide a drop of culture or microbial emulsion was placed using a plastic handle, and spread carefully so that the germs are evenly distributed without forming a clump and allowed to air dry the smear thus obtained.
When the smear is perfectly dry, it is fixed by heat.
+ GRAM coloring
- The smear was covered with gentian violet, for 1 minute.
- Gentian violet has been washed and replaced with potassium iodide for 30 seconds contact.
- After rejecting the potassium iodide solution, the slide was discolored by dropping the alcohol drop by drop on the surface, keeping it in an oblique position.
- When the alcohol flows colorless, the slide was quickly washed under running water.
- Recolouring with Fushine was carried out leaving it to act for 5 minutes then the slide was rinsed with water and allowed to air dry.
+ Microscopic observation:
Examine at the ×100 objective, immersion (with a drop of oil), with significant lighting (open diaphragm).
- Seeding and cultivation of biomaterials
From a bacterial suspension which corresponds to a turbidity of 0.5 McFarland of each bacteria studied (≈106 CFU/mL), we seeded the surfaces of the 2 pairs of blood agars using a sterile swab. (MHS) encrusted with fragments to be studied.
For Staphylococcus aureus is seeded on Mueller Hinton agar (HD). One dish in each pair was incubated for 6 hours at 37˚C and 5% CO2, the other dish in each pair was incubated for 24 hours at 37˚C and 5% CO2.
- Observations of the culture dishes
Macroscopic observation with a binocular magnifier (V.M.Z. 1 to 4 Japan, Olympus) of bacterial proliferation was carried out regularly after 6 h and 24 h of incubation.
Observation by optical microscope based on GRAM staining made it possible to visualize the presence or absence of bacteria and to differentiate them.
- Confirmation test for bacterial fixation on biomaterials by subculturing on culture media.
We scraped a portion of the culture on the surface of each substrate (tooth and stainless steel) with a calibrated loop of 1 µl and suspended with physiological water.
This suspension was readjusted to a concentration of 0.5 MacFarland and was subjected to different dilutions 1/10, 1/100 and 1/1000.
1 µl of each suspension prepared, was inoculated on different media specific to them.
Interpretive cultures were read after incubation in an oven at 37˚C for 24 hours.
- Bacterial adhesion
The adhesion of Staphylococcus aureus Méti S on dental fragments was compared to the one obtained on stainless steel fragments.
For example, the number of bacteria adhered as a function of the number of bacteria (CFU/ml) was reported for the two pre-incubated samples. The slopes of the lines obtained make it possible to determine the adhesion percentages.
- Bacterial count by optical dosing.
- The tooth fragments and the cultivated stainless-steel fragments are subjected to washing with sterile distilled water (EDS) in order to eliminate all the non-adhesive bacteria on the substrates.
- In each sterile tube containing 0.5 ml of 1× phosphate buffered saline (PBS), introduce the cultured material (tooth or stainless steel), Vortex for 5 minutes in order to release the bacteria fixed on the substrates.
- Following a procedure based on the protocols described by Christensen et al. (27). The suspension of bacteria was adjusted to an optical density (OD) of 0.5 to 610 nm. A 1:10, 1:100 and 1:1000 dilution was prepared with the adjustment of the bacterial suspension in tryptic soy broth (TBS). The contents of each tube were gently aspirated for the quantification of adhesive bacteria.
- The OD due to the resulting solution was measured at 560 nm.
- Optical dosing: is based on the Beer-Lambert law:
The form used is as follows:
+ A: Absorbance of the unitless solution
+ ε: Molar extinction coefficient in L × mol−1 × cm−1.
+ l: length of the tank through which the light passes in cm.
+ C: molar concentration in mol × L−1.
The absorbance measurement is given by a spectrophotometer which measures the optical density. The more a solution is concentrated, the more light has difficulty passing through the medium, which leads to an increase in the absorbance of the solution, the measurements obtained are expressed in OD (optical density), the solutions used in the optical assay are the same dilutions and the mother solution used in the dishes in the first method (bacterial count by culture).
3. Results
3.1. Confirmatory Tests for the Bacterial Strain
- -Culture on Chapmen: (Figure 1)
After 24 hours of incubation, a bacterial layer was observed all along the slope.
The consumption of mannitol by the strain allowed acidification of the medium and consequently the colored indicator turned from red to yellow, which confirms the présence of Staphylococci aureus.
- Biochemical catalase test: (Figure 2)
![]()
Figure 1. Culture of Staphylococci aureus ATCC 29213 on Chapmen medium after 24 h of incubation.
![]()
Figure 2. Appearance of the positive catalase test.
Bubbles appeared, which allowed us to conclude that staphylococci aureus are catalase positive.
- Gram staining:
Observation of the slide by an optical microscope (Leica microsystems DM1000) at the × 100 objective, showed cocci in clusters stained in purple (Gram+).
3.2. Observations with the Naked Eye of Bacterial Cultures on Biomaterials
- After 6 hours of incubation: (Figure 3)
No bacterial culture was observed on the plates encrusted with dental fragments and on the stainless steel fragments.
- After 24 hours of incubation: (Figure 4).
The dishes inoculated with Staphylococcus aureus showed the formation of a bacterial mat which covers the entire dish including the dental fragments (Figure 4(a)). While in the second dish, a decrease in proliferation around the stainless-steel fragments was observed with the appearance of a zone of inhibition of 2 to 3 mm.
![]() (a)
(a) ![]() (b)
(b)
Figure 3. Culture of staphylococcus aureus on HD agar encrusted with dental fragments and stainless-steel fragments after 6 hours of incubation.
![]() (a)
(a) ![]() (b)
(b)
Figure 4. Culture of Staphylococcus aureus on HD agar encrusted with dental fragments and stainless-steel fragments after 24 hours of incubation.
3.3. Culture of Staphylococci aureus on Chocolate Agar from the 6 h Culture
- Boxes from the culture on the stainless-steel fragments: (Figure 5)
Observation of the dishes confirms the results obtained beforehand, the dishes inoculated from the stock solution, the 1/10 and 1/100 dilutions represent a total absence of bacterial colonies.
- Boxes from the culture on dental fragments: (Figure 6)
Colonies were observed on the dishes as a function of the concentration of the inoculated suspension.
- Plates inoculated from the agar culture: (Figure 7)
Also represents colonies according to the concentration of the inoculated suspension, with a number greater than that found on the dishes inoculated from fragments of email.
3.4. Culture of Staphylococci aureus on Chocolate Agar from the 24 Hours Culture
- Boxes from the culture on the stainless-steel fragments: (Figure 8)
Even after 24 hours of incubation no colony was found on all the dishes.
- Boxes from the culture on the dental fragments: (Figure 9)
![]()
Figure 5. Dishes seeded from the 6 h culture on the stainless-steel fragments with different dilutions.
![]()
Figure 6. Plates seeded from the 6 h culture on the dental fragments with different dilutions.
![]()
Figure 7. Dishes inoculated from the 6 h culture on the agar with different dilutions.
![]()
Figure 8. Plates seeded from the 24 h culture on the stainless-steel fragments with different dilutions.
![]()
Figure 9. Plates seeded from the 24 h culture on the dental fragments with different dilutions.
An increase in the number of bacterial colonies was noticed on the different dishes.
- Dishes inoculated from the culture on the agar: (Figure 10)
A remarkable bacterial invasion on all the dishes inoculated from the culture carried out on agar.
3.5. Evaluation of the Adhesion Potential by Optical Assay (Table 1)
- Optical assay after 6 h of incubation gave zero results for both cultivated substrates
After 24 hours of incubation, we observed that the number of bacteria increases in the presence of dental fragments and the agar, so the count is zero in the presence of stainless-steel fragments.
3.6. Comparison between the Results of Enumeration from Cultures and Optical Assay
The two enumeration methods showed similar and correlated results for staphylococci after 6 h and 24 h incubation in the presence of dental fragments, stainless steel fragments and on agar.
Enumeration by both methods showed that the number of bacteria increased with incubation time in the presence of dental fragments, stainless steel fragments and on agar.
![]()
Figure 10. Plates seeded from the 24 h culture on the agar with different.
![]()
Table 1. Optical determination of the stock solution and of the different dilutions prepared after 24 hours of incubation in the presence of dental fragments, and stainless-steel fragments.
The results also showed that the bacteria count after 6 hours of incubation and in the presence of stainless-steel fragments is zero, which confirms the first results we obtained.
The synthesis of the stady’s results is represented in Table 2.
4. Discussion
In order to understand the mechanisms of bacterial adhesion on surfaces, we have cultured germs in contact with dental surfaces and stainless-steel surfaces. This allowed us to understand the bacterial adhesion and proliferation mechanisms using counting methods (by culture and by optical assay).
The bacteria we have chosen is present in the oral flora and was also available at the bacteriology laboratory at the University Hospital Center of Casablanca. The evaluation of the adhesion capacity of these germs was carried out by a bacterial count after 6 h and 24 h incubation.
We used two counting methods:
![]()
Table 2. Synthesis of the study’s results.
- A count by culture on a special medium by pigmentation of adherent bacteria and non-adherent bacteria,
- Enumeration by optical dosage of bacteria adhering to the substrates; for this count we used decreasing dilution solutions of the mother solution prepared for the 1/1000 dilution.
Observations with the naked eye of bacterial cultures on biomaterials:
- After 6 hours of incubation: No bacterial culture was observed on the plates encrusted with dental fragments and on the stainless-steel fragments.
- After 24 hours of incubation: The dishes inoculated showed the formation of bacterial covering the entire dish including the dental fragments. While in the second dish, a decrease in proliferation around the stainless-steel fragments was observed with the appearance of a zone of inhibition of 2 to 3 mm.
Similar studies by Elagli A. [22] have worked on the effects of titanium powder on seven bacteria generally found in dental plaque or in the gingival sulcus, they have shown that the titanium alloy has no inhibitory or stimulating on bacterial adhesion. The adhesion of bacteria to the material depends on the physicochemical and topographic surface properties of it.
The same study has shown that the culture dishes inoculated with Staphylococcus aureus, showed the formation of a bacterial carpet that covers the entire box including dental fragments. Whereas at the level of the second box a decrease in proliferation around the titanium fragments was observed with the appearance of a 1 mm inhibition zone. The same zone was observed in the present study around the stainless-steel fragments (2 to 3 mm) [23].
Culture of staphylococci aureus on chocolate agar from the 6 hours culture
- Boxes from the culture on the stainless-steel fragments:
Observation of the dishes confirms the results obtained beforehand, the dishes inoculated from the stock solution, the 1/10 and 1/100 dilutions represent a total absence of bacterial colonies.
- Boxes from the culture on dental fragments:
Colonies were observed on the dishes accordingly to the concentration of the inoculated suspension.
- Plates inoculated from the agar culture:
These plates also have a number of colonies according to the concentration of the inoculated suspension, with a number greater than that found on the dishes inoculated from fragments of email.
Culture of staphylococci aureus on chocolate agar from the 24 hours culture:
- Boxes from the culture on the stainless-steel fragments:
Even after 24 hours of incubation no colony was found on all the dishes.
- Boxes from the culture on the dental fragments:
An increase in the number of bacterial colonies was noticed on the different dishes.
- Dishes inoculated from the culture on the agar:
We observed a remarkable bacterial invasion on all the dishes inoculated from the culture carried out on agar.
After 24 hours of incubation and with a dilution of 1/100, there was an increase in bacterial colonies in the presence of dental fragments and the formation of bacterial colonies in the presence of agar, however we noticed a total absence of colonies in the presence of fragments in stainless steel with 1/100 dilution and with stock solution.
Similar studies [1] have shown that culturing cells on biomaterials in vitro after 6 hours of incubation is not sufficient for cells to adhere and proliferate on their substrates. Other studies have shown that the density of seeding influences the phenomena of adhesion and proliferation [2].
A study that aimed to compare bacterial adhesion to dental fragment and Titanium fragment has shown that after 6 hours of incubation with Staphylococcus aureus, the whole box was submerged by bacterial on the surface of the dental fragments. Also, on the agar containing titanium fragments, there was no bacterial proliferation. After 24 hours of incubation, a growth was observed on the entire agar surface containing dental fragments, only a few colonies were noticed on the agar inlaid with titanium fragments. The results also showed that with the other dilutions there were no bacterial colonies on the agars containing titanium fragments, after 6 h and after 24 h incubation, but on the surfaces of the titanium fragments there was no adhesion of these bacteria, instead they were inhibited. The study concluded that Staphylococcus aureus adheres and proliferates more easily on dental fragments on agar with the same dilution. However, it does not adhere and therefore does not proliferate in the presence of titanium fragments, even with the stock solution [23].
This disparity in results depends on the composition of the substrates and the nature of its surface state since several studies have shown the influence of these components on cell adhesion and proliferation phenomena [24] [25].
Optical microscopic examination of the bacteria showed from the first hours of culture that the presence of dental fragments and agar did not affect cell adhesion and spreading capacity. Numerous studies have shown that the porous or fibrillar structure of a material [26] [27] [28], its topography or its roughness [24] [25] [28] and its physicochemical properties [29] [30] [31] play a determining role in the phenomena of migration, adhesion and synthesis of the extracellular matrix.
The results obtained with the cultures of the germs in the presence of the dental fragments can be explained by the roughness of its surface state resembling that of the trabecular bone. Indeed, many in vivo studies have shown that rough surfaces allow better bone integration than smooth surfaces [26] [32] [33].
The dental fragments used in our study have rough surfaces and sometimes have reliefs, which could explain the proliferation of bacteria around the dental fragments contrary to the results obtained with the stainless-steel fragments. Similar results have been obtained with stainless steel having a smooth surface, where a significant inhibition of the proliferation of Staphylococus aureus has been observed [34].
Generally speaking, dental plaque buildup is much greater on rough surfaces than on smooth surfaces, such as metal alloys [35] [36]. Dental plaque not only adheres in larger quantities, but is also more difficult to remove when the surface of the material is uneven [37] [38]. As a matter of fact, the grooves and other surface defects lead to an increase in the potential surface to be colonized, and are places favorable to the creation of microbial niches [38].
The chemical composition of stainless steel is a factor that influences bacterial growth and adhesion. Indeed, stainless steel, commonly called stainless steel or stainless steel, is a steel alloy (containing less than 1.2% carbon) with more than 10.5% chromium. This alloy has a very different adhesion sensitivity than that of agar seeded by bacteria. Thus, the chemical composition of the material can influence the adhesion and colonization of bacteria on the adhesion surface.
Several researches are currently focused on chemical and topographic surface modifications of materials in order to develop surfaces with properties inhibiting bacterial adhesion [39] [40] [41].
Evaluation of the adhesion potential by optical assay:
Optical assay after 6 h of incubation gave zero results for both cultivated substrates.
Comparison between the results of enumeration from cultures and optical assay:
The two enumeration methods showed similar and correlated results for staphylococci after 6 h and 24 h incubation in the presence of dental fragments, stainless steel fragments and on agar.
Enumeration by both methods showed that the number of bacteria increased with incubation time in the presence of dental fragments, stainless steel fragments and on agar.
The results also showed that the count of the bacteria after 6 hours of incubation and in the presence of the three substrates is very low to zero, which confirms the primary results that we obtained.
After 24 hours of incubation we found that the number of bacteria increases in the presence of dental fragments and agar. In contrast, the number of bacteria increases only with Staphylococcus aureus in the presence of the stainless-steel fragments.
The adhesion and proliferation of bacteria depend on the types of bacteria, the conditions of cell culture but also on the properties of the biomaterials used. The surface topography of a biomaterial affects not only adhesion but also the migration and proliferation of bacterial strains [42] [43].
An in vitro study that aimed to evaluate the ability of Staphylococcus aureus, Staphylococcus epidermidis, Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa to adhere to and to form biofilms on the surface of five orthopaedic biomaterials, viz., cobalt and chromium, highly cross-linked polyethylene, stainless steel, trabecular metal, and titanium alloy, have shown that the highest level of adherence was observed on highly cross-linked polyethylene, followed by titanium, stainless steel, and trabecular metal, with the lowest occurring on the cobalt-chromium alloy. Among the bacterial strains tested, the ability for high adherence was observed with S. epidermidis and K. pneumoniae followed by P. aeruginosa and E. coli, whereas S. aureus showed the least adherence [44].
Stainless steel (316L) is a metallic biomaterial with a sensible biocompatibility and simple to machine; consequently, it is broadly utilized for orthopedic, cardiovascular and craniofacial applications because of its good corrosion resistance and formability [45].
The adhesion of bacteria on material surfaces is governed by many factors, including bacterial characteristic (e.g., hydrophobicity, surface charge), surface properties (e.g., roughness, wettability) and environment condition (e.g., pH, temperature) which involve the physicochemical and the molecular interactions [46].
Studies have showed that many factors affect the initial attachment of organisms to inert substrata, and their subsequent retention or removal/detachment, including the physical and chemical nature and location of the substratum, the type of organic material and microorganisms potentially fouling the surface, and the nature of the interface (solid-liquid in the body; solid-air on environmental surfaces) [47].
The composition and structure of surface biofilm depend on the biomaterial’s localization. Moreover, the flow of saliva is also an important factor which may change the quantity and quality of biofilm. During saliva flow, the components of EPS can be changed, which may influence the adhesiveness of the biofilm to the surface. Biomaterials exposed to a high flow of saliva (where high shear forces occur) are less susceptible to biofilm formation [48]. On the other hand, surfaces with locally high nutrient content for microorganisms are more susceptible to colonization—for example, between denture elements. The presence of biofilm on dental metallic biomaterials in the oral cavity is related to numerous processes of its surface destruction, such as corrosion and friction. In the case of the second phenomenon (friction), the wear process of teeth and biomaterial [21] [44].
The experimental results showed also that the bacterial attachments are in increasing order when the surface roughness of biomaterials increases but at the same time the bacterial inhibitions are in decreasing order. Which can explain the difference of adhesion between dental and stainless-steel surfaces in our study [45].
Rough surfaces of orthodontic archwires for example provide opportunities for bacterial adhesion by increasing the surface area, providing suitable niches for bacteria and impairing bacterial colony dislodgment. Where the biofilm first develops within the valleys of uneven surfaces by irreversible attachment of planktonic pioneer bacteria, smoothing the rough regions [49].
In addition, changes in Surface Roughness of greater than 0.1 mm influence the contact angle, there by changing the surface free energy values, which comprise the second surface characteristic affecting bacterial adhesion to orthodontic materials [50].
5. Conclusion
This study aimed to understand factors associated with bacterial biofilms in dental pathology. We investigated the adhesion rate of Staphylococcus aureus to dental and stainless-steel surfaces. It showed that this germ has different behaviors in contact of the two surfaces and that consequently its capacity of adhesion was different to the two surfaces. We can conclude that this bacterium was able to adhere and proliferate with the tooth surfaces, whereas in contact with the stainless surfaces, an inhibition of this adhesion was observed. This confirms that surface characteristics especially its roughness have a direct influence on the adhesion of bacteria on it. However, to understand the aspects of bacterial pathogenic process and the entire phenomenon an in vivo study must be conduced.