Crystal and Physiochemical Properties of 2-Aminoethanaminium 2-(Ethoxycarbonyl)-4,6-Dinitrophenolate ()

1. Introduction
Proton transfer interactions between electron donor and electron acceptor molecules absorb radiation in the visible region leading to the formation of intensely colored charge transfer complexes [1] [2] [3] [4] [5] .
Based on the concepts of the molecular and crystal engineering, the organic molecules exhibit many possibilities to tailoring the substances with desired properties through optimization of the microscopic hyper polarizabilities and the incorporation of the molecules in a crystalline lattice [6] [7] [8] [9] .
3,5-dinitrosalicyclic acid is widely used in reducing sugar quantification [10] as well as its proton transfer investigation with many amines has been studied [11] , no studies state the properties of these molecular salts in reducing sugar quantification.
In the present study, the proton transfer of ethyl ester of 3,5-dinitrosalicylic acid and ethylenediamine has been carried out and both of crystal and physicochemical product properties are outlined. It was expecting stoichiometry 2:1 acid to base but the X-ray crystallography confirmed the 1:1 molar ratio of the salt and it showed superior properties in reducing sugar quantification as compared to 3,5-dinitrosalicylic acid DNS [10] .
2. Experimental Procedure
2.1. Preparation of Ethyl 3,5-dinitrosalicylate
Ethyl ester of DNS, 3,5-dinitro ethylsalicylate, was prepared according to both of the described proceures [12] and the described procedures [13] using ethyl salicylate instead of methyl salicylate, the products were identical in spectral analysis as well as physical propertie, pale yellow solid compounds, δ = 1.3 (t, CH3 ester), 4.2 (q, CH2 ester), 8.4 - 8.6 (m, CH aromatic), m.p. 73˚C, crystallized from ethanol-water 1:1 v/v. The purity was tested by thin layer chromatography (TLC).
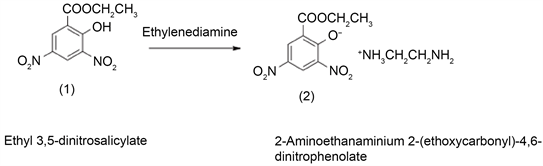
2.2. Preparation of 2-Aminoethanaminium 2-(ethoxycarbonyl)-4,6-dinitrophenolate Salt
Ethyl 3,5-dinitrosalicylate (1) (1 mmole) is dissolved in 10 ml boiled absolute ethanol and ethylenediamine (4 mmole) is added, the mixture was boiled for five minutes. The yellow solid product formed (2) was filtered out, dried and recrystallized from water. Yellow solid, m.p 192˚C, Scheme 1.
2.3. Single Crystal Growth
A saturated DMF-water, dimethylformamide-water, solvent mixture of 2-Aminoethanaminium 2-(ethoxycarbonyl)-4,6-dinitrophenolate salt (AED) was stirred for half an hour and filtered through quantitative whatmann 41 grade filter paper, the clear solution was kept undisturbed in a clean environment for one week.
2.4. Examination in Reducing Sugar Quantification
Following the same procedures have been described by Miller and a recently published work [10] [14] [15] [16] [17] .
3. Results and Discussion
3.1. Nuclear Magnetic Resonance Studies (NMR)
The 1H and 13C NMR spectra of 2-Aminoethanaminium 2-(ethoxycarbonyl)-4,6-dinitrophenolate (AED) were recorded using the BRUKER AC 400MHz spectrophotometer with TMS as the internal reference standard and DMSO as a solvent.
δ = 1.3 (t, CH3 ester), 4.2 (q, CH2 ester), 3.7 (s, CH2 adjacent
), 7.8 (s, +NH3), 8.4 - 8.6 (m, CH aromatic), 2.7 (s, CH2 adjacent NH2), 2.9 (s, NH2), 13C NMR in DMSO, 14.15 ppm for CH3 ester carbon, 60.01 ppm for CH2 ester carbon, 165.15 ppm for C=O ester, 36.66 ppm for CH2 ethylenediamine carbon, 123.78 - 139.98 ppm for aromatic carbons and 167.17 ppm for aromatic carbon attached to ester group.
The title crystal shows eight proton signals indicating the presence of eight different proton environments in the aminium crystal. The broad hump appearing at δ 7.8 ppm is assigned to the highly deshielded
protons. The intense singlet signal appearing at δ 8.4 - 8.6 ppm has been assigned to aromatic protons. The intense signal at 3.7 ppm is assigned to the methylene group beside the highly shielded aminium group and another signal appeared at 2.7 ppm for methylene adjacent to the free amino group.
3.2. Fourier-Transform Spectroscopy (FT-IR)
The characteristic vibrational frequencies of the functional groups of the aminium salt are identified from the fourier transform infrared (FT-IR) spectrum recorded in the range of 4000 - 400 cm−1 employing Shimadzu Affinity 1S FT-IR spectrometer. The formation of charge transfer complex during the acid-base interaction of ethylenediamine with ethyl 3,5-dinitrosalicylate is strongly evidenced through the realization of important bands in the complex salt. The absorption at 3194 cm−1 is due to the +N-H stretching vibration. The absorption band at 3060 cm−1 corresponds to aromatic C-H asymmetric stretching vibration. The broad absorption bands in the region 3400 to 3600 cm−1 are due to NH2 asymmetric and symmetric stretching vibration. The absorptions at 1535 and 1359 cm−1 confirm the asymmetric and symmetric stretching vibrations of NO2 group respectively. The C=O stretching vibration is observed at 1680 cm−1.
3.3. Single Crystal X-Ray Diffraction Studies (XRD)
A yellow chip crystal having approximate dimensions of 0.500 × 0.300 × 0.300 mm was mounted on a glass fiber. All measurements were made on a Rigaku SCX mini diffractometer using graphite monochromated Mo-Kα radiation. The crystal-to-detector distance was 52.00 mm.
The crystal structure analyses reveal it is crystallized in monoclinic crystal structure with centrosymmetric space group C2/c and the unit cell parameters are a = 15.289(2) Å, b = 20.755(3) Å and c = 20.545(3) Å. The crystal structure has been already deposited in Cambridge Crystallographic Data Center coded CCDC 1441586, Figure 1 represents the molecular ORTEP diagram for the aminium salt [14] .
Some of the crystal data are listed (Table 1).
3.4. Ultraviolet-Visible spectroscopy (UV-Vis)
The optical transmission spectrum of the aminium salt was recorded in the region 400 - 1000 nm employing a Shimadzu 1061 UV-Vis spectrophotometer in
![]()
Figure 1. The molecular ORTEP diagram for 2-aminoethanaminium 2-(ethoxycarbonyl)-4,6-dinitrophenolate.
solution using DMSO as the solvent. The percentage of transmittance was around 100 in the region between 460 and 1000 nm. Hence, this crystal can be used for the suitable optical applications due to its wide transparency range in the part of visible region above 460 nm.
3.5. Thermal analysis (TG/DTA)
TG/DTA analysis, the sample is analyzed between 25˚C - 600˚C at a heating rate 10 K/min in nitrogen atmosphere. The DTA reveals exactly same changes shown by the TGA. From thermo gravimetric curve it is inferred that the material decomposes immediately after melting into gaseous products. The material exhibits sharp weight loss at 192.47˚C and below this temperature no significant weight loss. The endothermic peak appears at 190.97˚C in DTA corresponds to the melting point of the compound and another sharp exothermic peak at 268.84˚C may be attributed to decomposition temperature of the aminium salt.
4. Conclusion
The organic molecular charge transfer salt 2-Aminoethanaminium 2-(ethoxycarbonyl)-4,6-dinitrophenolate (AED) was synthesized and the single crystals were grown by slow evaporation solution growth technique using DMF-water as the solvent. FT-IR, 1H and 13C NMR spectral techniques confirm the molecular structure of the aminium salt. The single crystal XRD study reveals that it crystallizes in monoclinic crystal structure with centrosymmetric space group C2/c and the unit cell parameters are a = 15.289(2) Å, b = 20.755(3) Å and c = 20.545(3) Å. UV-Vis transmittance study shows that the attained percentage of transmission was around 100% for the aminium salt in the region between 460 - 1000 nm. The title crystal is a good candidate for suitable optical applications as well as its superior properties in reducing sugar quantification.
Acknowledgements
I express my deep sense of gratitude to the Indian Council for Cultural Relations for both funding me and giving me the opportunity to do a research in India and also my great appreciation to the Center of Excellence in Drug Discovery at NFDD Complex, Saurashtra University Campus, Rajkot, India, for all facilities I have been supported.