Ethnobotany, Pharmacology and Phytochemical Investigations of the Seeds of Pentaclethra macrophylla Benth (Mimosaceae) ()
1. Introduction
Plants have been used in traditional medicine worldwide for the treatment of diseases and many drugs have been developed from their chemical constituents. This is also the case for species of the genus Pentaclethra belonging to the Mimosaceae family comprising plants widely distributed in Africa and South America [1]. This genus contains only three species including Pentaclethra eetveldeana, Pentaclethra macroloba and Pentaclethra macrophylla [2]. Pentaclethra macrophylla can have 21 m in high and approximatively 60 cm in diameter and is known as the African oil bean tree. It is mostly found in the forests of the Eastern and Western regions of Nigeria and in some regions of Senegal, Angola, Cameroon and Gabon [3]. Plants of the genus Pentaclethra have an ethnopharmacological background in the African traditional medicine where they are used for the treatment of several diseases such as itch, gonorrhoea, diarrhoea, small pox and infertility [4] [5]. Previous phytochemical studies revealed that plants of the genus Pentaclethra are a rich source of secondary metabolites such as saponins, tannins, alkaloids, flavonoids and monoglycerides [6]. Previous chemical investigation of the seeds of P. macrophylla led to the isolation of two diterpernoids, secopentaclethrolide and secopentaclethroside; one alkaloid, caffeoylputrescine and one glycerol derivative, glyceryl monotetracosanoate [7]. Recent pharmacological studies on stem bark, and leaves of this plant revealed its antinociceptive [4], antidiarrheal [8], antimicrobial [9] [10], and hepatoprotective [11] activities. In the course of our continuing search for bioactive secondary metabolites from medicinal plants growing in Cameroon [12] [13] [14], we undertook the phytochemical investigation of the n-butanol soluble fraction from the ethanol extract of the seeds of P. macrophylla leading to the isolation and structure elucidation of an inseparable mixture of two new aromatic monoterpene glycosides together with six known compounds (Figure 1).
2. Ethnobotanical and Traditional Uses of Pentaclethra macrophylla
Pentaclethra macrophylla popularly known as African oil bean is a member of the Leguminosae family. A decoction of fermented extract of seeds of this plant has been known to be effective in the management of malnutrition, gastrointestinal disorders and dental caries [15]. The bark, fruits, seeds and the leaves are used as anthelmintics, for gonorrhea, convulsion, and as analgesic [16] [17] [18]. The extracted oil from the seeds is used as remedy against pruritus, worms, and dysentery [19] [20] [21]. Moreover, the seeds are used for the treatment of diabetes in Nigerian folk medicine [22]. In addition, it is used for the treatment of itching and pain in animals and in man [23].
3. Pharmacological Properties of Pentaclethra macrophylla Seeds
3.1. Antiulcerogenic Activity
The antiulcerogenic potentials of aqueous extract of fermented P. macrophylla
![]()
Figure 1. Structures of compounds (1-7).
seeds were studied using acetic acid, aspirin, ethanol, indomethacin and pyloric ligation of ethanol induced ulcer models at the doses of 400 and 800 mg/kg body weight. Omeprazole at 5 mg/kg was used as a standard reference drug. The different doses of the extract and the reference drug decreased significantly (p < 0.05) the ulcer parameters in a dose-dependent manner in all the ulcer models. Moreover the result of the acute toxicity test showed that the extract did not cause any mortality of the animals possessing 5000 mg/kg body weight. Therefore, the enhanced cessation of gastric erosions could be attributed to the synergistic role of biochemicals and microbiomes residents in fermented aqueous extract of P. macrophylla seeds; suggesting that a decoction of fermented aqueous extract of seeds of this plant could be employed in ethnomedicine for the treatment of peptic ulcer [15].
3.2. Antiinflammatory and Analgesic Activities
The leaves and seeds of P. macrophylla extracts were tested for analgesic and anti-inflammatory activities using mice with in-vivo and in-vitro experimental models. The extracts at 30 and 60 mg/kg exhibited analgesic activity and anti-inflammatory property using the flick and hot plate tests, acetic acid induced writhing test; and leucocyte counts, pulmonary oedema and oedema paw of mice in a dose-dependent manner. This result therefore explains and justifies the ethnomedical uses of seeds of P. macrophylla in the treatment of itching (inflammatory response) and pain in animals and in man [23].
3.3. Antimicrobial Activity
The antimicrobial efficacy of the ethanol, methanol and water extracts of P. macrophylla seeds was evaluated against seven selected pathogens including Escherichia coli,Staphylococcus aureus,Pseudomonas aeruginosa,Klebsiella species,Salmonella typhi,Aspergillus niger and Candida albicans using paper disc and hole diffusion methods. The results showed that the growths of test organisms were inhibited by extracts used and the minimum inhibitory concentration of the extracts ranges between 62.5 - 250 mg/ml on the tested isolates. However, the antimicrobial potency of P.macrophylla seeds was more prominent against bacterial isolates than fungal isolates [24].
3.4. Antioxidant Activity
The antioxidant capacity of the extracts from three samples (raw, dried and autoclaved) of P. macrophylla seeds oil was evaluated using 2,2-diphenyl-1-picryphydrazyl (DPPH). For each sample three different solvents were used for extraction (70% ethanol, 80% acetone and acidic 70% acetone). Globally the 70% ethanol extracted samples showed the greatest antioxidant activity in the DPPH free radical assay [25].
3.5. Anti-Hyperlipidemic Activity
The antihyperlipidemic effects of the P. macrophylla seeds in high fat diet and streptozotocin-induced diabetic wistar rats were evaluated. Blood glucose and Lipid prolife of animals were analyzed 6 days after STZ injection and feeding to confirm hyperlipidemia and hyperglycemia. 50% rat feed was substituted with 50% of the various processed P. macrophylla seeds (raw, 1st cooking, 2nd cooking, fermented and fermented and salted) and used to feed the animals [22]. Metformin was administered daily by intra-gastric gavages for 2, 4, 6 and 8 weeks. Treatment of high fat diet and streptozotocin-induced diabetic rats with various processed P. macrophylla seeds over a period of 8 weeks significantly (p < 0.05) reduced the levels of plasma, total cholesterol, triglycerides and LDL-cholesterol and increased HDL-cholesterol compared to rats not feed with various processed P. macrophylla seeds. The various processed P. macrophylla seeds also exhibited hypolipidemic activities in high fat diet and streptozotocin-induced diabetic wistar rats for the 8-weeks of treatment. They could be used as source of functional foods providing essential micronutrient preventing progression to cardiovascular diseases [22].
4. Materials and Methods
4.1. General Experimental Procedures
Melting points were recorded on SMP20 apparatus. 1H and 13C NMR, COSY, HSQC and HMBC spectra were performed in deuterated solvents (CD3OD; DMSO-d6) on a Bruker AVANCE 500 and 600 spectrometers (Bruker, Germany) at 500 MHz/125 MHz and 600 MHz/150 MHz. All chemical shifts (δ) are given in ppm units with reference to tetramethylsilane (TMS) as internal standard, while the coupling constants (J values) are given in Hertz (Hz). Positive ion mode ESI mass spectra were carried out on an Agilent 6210 ESI-TOF mass spectrometer (Agilent Technologies, Santa Clara, CA, USA) and on an Agilent Technologies LC/MSD Trap SL (G2445D SL). Purification of fractions and sub-fractions were carried out by column chromatography, Medium-Pressure Liquid Chromatography (MPLC) system [Büchi pump manager C-615, Büchi pump module C-605, Büchi column (460 × 15 mm and 230 × 15 mm)] using silica gel 60 (0.006 - 0.035 mm, 0.063 - 0.200 mm and 0.04 - 0.063 mm) and sephadex LH-20. The following solvent systems were used: MeOH for Sephadex column chromatography, mixtures of Hexane-EtOAc, EtOAc-MeOH and EtOAc-MeOH-H2O for silica gel column chromatography, EtOAc-MeOH and EtOAc-MeOH-H2O for MPLC. TLC was carried out on precoated silica gel 60 F254 (Merck) plates developed with Hexane-EtOAc, EtOAc-MeOH and EtOAc-MeOH-H2O. The spots were visualized under UV light (254 and 365 nm) of lamp multiband (Model UVGL-58 Upland CA 91786, U.S.A) and by spraying with 10% H2SO4 followed by heating for 10 min.
4.2. Plant Material
The seeds of Pentaclethra macrophylla were collected in Lo’Obiyeng village (with the location 2˚57'35"N, 11˚7'23"E), Mvilla division, South Region of Cameroon, in June 2016. The plant material was identified by Mr NANA Victor, botanist at the Cameroon National Herbarium, Yaoundé (Cameroon) in comparison with a voucher specimen deposited on number 30002/HNC.
4.3. Extraction and Isolation
The seeds of P. macrophylla were air-dried, ground to give 5 Kg of powder. This powder was extracted by maceration with 8 L of ethanol (95%) at room temperature three times (each time for 24 h). After filtration, the solvent was removed by evaporation under reduced pressure to yield a crude ethanol extract (420 g). Part of this extract (400 g) was suspended in distilled water (500 mL) and successively extracted with ethyl acetate (800 mL) and n-butanol (750 mL). The resulting soluble fractions were concentrated to dryness under reduced pressure to give the ethyl acetate (55 g) and n-butanol (49 g) fractions respectively. Part of the n-butanol fraction (45 g) was submitted to silica gel column chromatography, using EtOAc and a gradient of MeOH in EtOAc ranging from (1:0) to (1:1) to afford three main sub-fractions (A-C). Recrystallization and filtration of sub-fraction A (1 g) yielded compound 5 (100 mg) and the resulting filtrate was chromatographed on silica gel column using isocratic elution with EtOAc to give two sub-fractions A1 and A2. Sub-fraction A1 (0.3 g) was chromatographed over silica gel column eluted by EtOAc to afford compound 6 (15 mg). The sub-fraction A2 (0.2 g) was also subjected to silica gel column chromatography using EtOAc as eluent, yielding compound 2 (20 mg). Sub-fraction B (2 g) was chromatographed on silica gel column eluted with a mixture of EtOAc-MeOH (19:1) to yield a mixture of white powder which was further purified by Medium Pressure Liquid Chromatography (MPLC) on normal phase (silica gel: 6.3 - 35 μm) eluted with EtOAc-MeOH (98:2) to give compound 3 (20 mg). Sub-fraction C (8 g) was submitted to silica gel column chromatography eluted with EtOAc-MeOH-H2O (8:2:1) to afford three sub-fractions C1-C3. Sub-fraction C3 (500 mg) was purified by MPLC on normal phase, eluted with mixture of EtOAc-MeOH-H2O (8:1.8:0.2) to yield compound 7 (15 mg) and compound 1 (20 mg) which was finally revealed as a mixture of two inseparable compounds (1a and 1b) after using several purification techniques. Sub-fraction C2 (2.5 g) was recrystallized several times with EtOAc-MeOH (80-20) to give 600 mg of compound 4.
4.4. Methodology of Antimicrobial Assay
The antimicrobial assay of extracts and some compounds was performed using a microdilution method [26] to determine their minimum inhibitory concentrations (MIC) against five bacterial strains (Staphylococcus aureus (ATCC 1026), Enterococcus faecalis (ATCC 29212), Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC74117), and one isolate: Proteus mirabilis) and three yeasts (One strain of Candida albicans ATCC 9028, and two isolates: Candida glabrata and Candida dubiniensis). Ciprofloxacin and ketoconazole were used as references. Briefly, the test sample and the selected antibiotic were dissolved in dimethylsulfoxide-Mueller Hinton broth (DMSO-MHB) and dimethylsulfoxide-Sabouraud Dextrose broth (DMSO-SDB). The solution obtained was then added to MHB and SDB and serially diluted twofold in 96-well microplates to give a final concentration range of 2 to 1024 µg/mL for extracts and from 0.5 - 128 µg/mL for pure compounds and references. One hundred microliters of inoculums prepared in MHB at a concentration of 1.5 × 106 CFU/mL were then added, even for SDB. The plates were covered with a sterile plate sealer and then agitated with a shaker to mix the contents of the wells and incubated at 37˚C. The final concentration of DMSO was less than 2.5%, and DMSO did not affect the microbial growth. Wells containing only MHB or SDB, 100 µL of any inoculum and DMSO at a final concentration of 2.5% served as the negative control. The MICs of samples were detected after 18 h, following addition of 40 µL of INT 0.2 mg/mL and incubation at 37˚C for 30 min. Viable bacteria reduced the yellow dye to pink. The MIC was defined as the lowest sample concentration that prevented the colour change and that resulted in the complete inhibition of microbial growth. Each assay was repeated three times independently. For the determination of the minimum bactericidal concentration (MBC), a portion of the liquid (5 μL) from each well that showed no change in colour was plated on MHB and incubated at 37˚C for 24 h. The lowest concentration that yielded no growth after this sub-culturing was taken as the MBC. Even, the low concentrations which induced an absence of turbidity at the bottom of the well after incubation were noted as the MFC.
5. Results and Discussion
The structures of compounds 2 - 7 were determined on the basis of their spectroscopic and mass spectrometric data in comparison with those reported in the literature as comososide (2) [27], secopentaclethroside (3) [7], caffeoylputrescine (4) [28] [29], β-sitosterol-3-O-β-D-glucopyranoside (5) [30], 2-hydroxymethyl-5-(2-hydroxypropan-2-yl)phenol (6) [31], and sucrose (7) [32].
Compound 1 was obtained as colorless oil. Despite the apparent homogeneity of its spot on TLC, it was deduced to be a mixture of two compounds from its spectroscopic analysis. After repeated column chromatography and MPLC separation using different adsorbents (silica gel, Sephadex LH-20, RP-18) no separation was attempted. The negative mode HRESI-MS showed two quasi-molecular ions peaks at m/z 487.1841 [M-H]− (calcd. for C22H31O12: 487.1821) and at m/z 357.1202 [M-H]− (calcd. for C16H21O9: 357.1191) corresponding to the molecular formulas of C22H32O12 and C16H22O9 for compounds 1a and 1b, respectively. This was also confirmed by the positive mode HRESI-MS which showed two ion clusters at m/z 511.1759 [M+Na]+ (calcd. for C22H32NaO12: 511.1786 corresponding to C22H32O12) and at m/z 381.1157 [M+Na]+ (calcd. for C16H22NaO9: 381.1156 corresponding to C16H22O9). The 1H-NMR spectrum showed signals of two ABX-spin systems of aromatic protons at δH 7.32 (d, J = 8.0 Hz, H1a-6), 7.16 (dd, J = 1.5; 8.0 Hz, H1a-5), 7.37 (d, J = 1.5 Hz, H1a-3) and at δH 7.77 (d, J = 8.0 Hz, H1b-6), 7.07 (dd, J = 1.6; 8.0 Hz, H1b-5) and 7.09 (d, J = 1.6 Hz, H1b-3), characteristic of two 1,2,4-trisubstituted phenyl rings [31]. Signals of two pairs of singlets at δH 1.58 (H1b-9), 1.68 (H1b-10), and 1.54 (s, H1b-9/H1b-10) assignable to four methyl groups are also found, suggesting the presence of two isopropanolic hydroxyl groups [33]. The presence of two oxygenated methylene protons was evidenced by signals at δH 5.28 (d, J = 12.4 Hz, H1a-7a) and 5.23 (d, J = 12.4 Hz, H1a-7b). This spectrum also showed two anomeric protons at δH 4.93 (o) and 4.35 (d; J = 7.6 Hz; H1b-1’) suggesting the presence of two β-glucopyranosyl moieties [34] (Table 1). The above data suggested that, the aglycone parts of the two aromatic compounds are derivatives of 2-hydroxymethyl-5-(2-hydroxypropan-2-yl)-phenol [27] [31]. The 13C NMR spectrum exhibited signals for two pairs of methyl groups at δC 30.4 (C1a-9), 30.3 (C1a-10) and 29.4 (C1b-9), 26.8 (C1b-10); two oxygenated aromatic carbons at δC 155.3 (C1a-2) and at δC 161.0 (C1b-2); four substituted aromatic carbons [δC 113.5 (C1b-1), 123.5 (C1a-1), 151.6 (C1a-4), 151.9 (C1b-4)]; six methine aromatic carbons [δC 129.0 (C1a-6), 118.3 (C1a-5), 111.9 (C1a-3) and 129.7 (C1b-6), 116.7 (C1b-5), 114.0 (C1b-3)]; two oxygenated sp3 quaternary carbons at δC 71.6 (C1a-8) and at δC 78.6 (C1b-9) and two anomeric carbons at δC 101.5 (C1a-1’) and at δC 98.3 (C1b-1’). From the 1H and 13C NMR data (Table 1), it was evident that 1 was a mixture of two glycosylated aromatic monoterpenoids. The difference between the two aglycone parts was the presence of only one oxymethylene group at δC 61.3 (C1a-7) in compound 1a and a carboxyl group at δC 174.5 (C1b-7) in compound
![]()
Table 1. 1H NMR (600 MHz) and 13C NMR (150 MHz) data of compounds 1a and 1b, CD3OD, δ in ppm multiplicity and J (Hz) in brackets.
O: Overlapped signal.
1b. The position of this carboxyl group was evidenced by the HMBC correlation depicted between the signal at δH 7.77 (d, J = 8.0 Hz, H1b-6) and C1b-7 (δC 174.5). The different linkage sites of the sugar moieties to the aglycones were determined by the HMBC cross-peak correlations depicted between the anomeric protons at δH 4.93 (H1a-1’), 4.35 (H1b-1’) and carbons at δC 155.3 (C1a-2) and 78.6 (C1b-8), respectively (Figure 2). Additionally, the 13C NMR spectrum exhibited six signals including a methyl at δC 26.4 (C1a-6”), two methylene groups at δC 45.3 (C1a-4”, C1a-2”), an hydroxymethine group at δC 69.5 (C1a-3”), an ester carbonyl at δC 171.5 (C1a-1”) as well as a carboxyl group at δC 175.2 (C1a-5”) characteristic of a 3-hydroxy-3-methylglutaric acid moiety [35]. The location of
![]()
Figure 2. Some important HMBC correlations of compounds 1a and 1b.
the 3-hydroxy-3-methylglutaric acid moiety on C1a-7 was evidenced from the HMBC correlation depicted between the proton at δH 5.23 (d, J = 12.4 Hz, H1a-7) and the carbon at δC 171.3 (C1a-1”). On the basis of the above data, the structures of compounds 1a and 1b were elucidated as two new naturally occurring aromatic monoterpenoids to which the trivial names pentamacrophyllosides A (1a) and B (1b) were given.
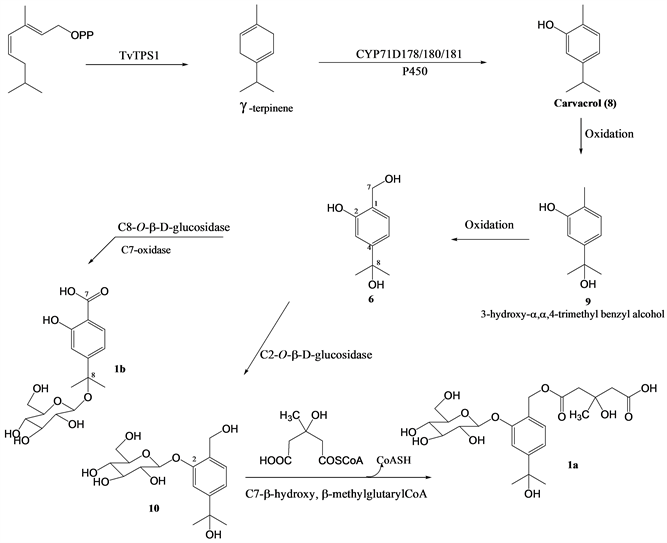
Compounds 1a and 1b are aromatic monoterpenoids and could biosynthetically derive from geranyl diphosphate. γ-terpinene synthase (TvTPS1) which is a member of the monoterpene synthase family could produce γ-terpinene through cyclization of geranyl diphosphate (Scheme 1). Enzymes such as CYP71D178, CYP71D180 and CYP71D181 belonging to the cytochrome P450 monooxygenases are also involved in further modifications of γ-terpinene backbone to yield carvacrol (8) [36]. Oxidation of carvacrol could lead to 3-hydroxy-α,α,4-trimethyl benzyl alcohol (9) previously isolated from Lavandula gibsonii [33] and compound 6 [31], respectively. The action of C8-O-β-D-glucosidase on 6 could yield pentamacrophylloside B (1b). The enzyme C2-O-β-D-glucosidase could then convert 2-hydroxymethyl-5-(2-hydroxypropan-2-yl)phenol (6) to mariaterpenoside A (10) previously isolated from Silybum marianum [31] while the esterification of compound 10 by β-hydroxy, β-methylglutaryl-CoA could afford pentamacrophylloside A (1a) (Scheme 1).
Phytochemical studies have previously been carried out only on stem bark and roots of other species of the genus Pentaclethra. Therefore, previous studies on stem bark of P. macroloba,and P. eetveldeana led to the isolation of saponins [2] [37] [38], phenolic compounds [39] [40] [41], and fatty acid derivatives [37] [39]. Furthermore, tannins were isolated from stem bark of P. macroloba and P. macrophylla [39] [40] [41]. Previous pharmacological studies on other species of the genus Pentaclethra particularly on P. macroloba seeds showed its antioxidant, and antimicrobial activities [42] [43]. Compounds 3 and 4 were previously isolated from P. macrophylla seeds [7] . Concerning caffeoylputrescine (4), it was the first secondary metabolite isolated from a plant of the genus Pentaclethra [44]. Its isolation from the fruits of P. macrophylla during our investigationis not surprising since it was reported that putrescine, spermidine, spermine and their derivatives are the main polyamines present in plants, involved in the regulation of diverse physiological processes such as flower development, embryogenesis,
Scheme 1. Proposed biogenetic pathways to the formation of compounds 1a and 1b from geranylpyrophosphate.
organogenesis, senescence, and fruit maturation and development [45]. Aromatic monoterpenoids comososide (2) and 2-hydroxymethyl-5-(2-hydroxypropan-2-yl)phenol (6) were previously obtained from Curcuma comosa (Zingiberaceae) [27] and Silibum marinum (Asteraceae) [31], respectively. To the best of our knowledge this is the first report on the isolation of this class of compounds from a plant of the genus Pentaclethra.
Although the n-BuOH extract showed a moderate activity against Candida albicans ATCC 9028, Enterococcus faecalis (ATCC 29212) and Proteus mirabilis with MIC values of 256, 512 and 512 μg/mL, respectively (Table 2), the isolated compounds were not active against the five tested bacterial and three yeasts strains compared to ciprofloxacin and ketoconazole used as references, respectively.
6. Spectroscopic Data of Compounds 1 - 7
Pentamacrophylloside A (1a): Colorless oil; 1H NMR data (600 MHz, CD3OD) and 13C NMR data (150 MHz, CD3OD) see Table 1; HR-ESI-MS (negative ion mode) m/z 487.1841 [M-H]− (Calcd. for molecular formula C22H31O12: 487.1821) and HR-ESI-MS (positive ion mode) m/z 511.1759 [M+Na]+ (Calcd for C22H32NaO12: 511.1786).
![]()
Table 2. The minimum inhibitory concentration (µg/mL) of extracts and some isolated compounds against the tested microorganisms.
-: ≥1024 µg/mL;Ca: Candida albicans, Cg: Candidaglabrata,Cd: Candida dubiniensis, Ec: Escherichia coli, Sa:Staphylococcus aureus, Pa:Pseudomonas aeruginosa, Ef:Enterococcus faecalis, Pm:Proteus mirabilis.
Pentamacrophylloside B (1b): Colorless oil; 1H NMR data (600 MHz, CD3OD) and 13C NMR data (150 MHz, CD3OD) see Table 1; HR-ES-IMS (negative ion mode) m/z 357.1202 [M-H]− (Calcd for C16H21O9: 357.1191) and HR-ESI-MS (positive ion mode) m/z 381.1157 [M+Na]+ (Calcd for C16H22NaO9: 381.1156).
Comososide (2): Yellow oil; 1H NMR (CD3OD, 600 MHz): δH = 7.27 (d, J = 1.0 Hz, H-3), 7.09 (d, J = 7.8 Hz, H-6), 7.04 (dd, J = 1.0, 7.8 Hz, H-5), 4.91 (d, 7.3, H-1’), 3.84 (dd, J = 1.9; 12.0 Hz, 1H, H-6’), 3.70 (dd, J = 12.0 Hz, 4.9 Hz, H-6’), 3.49 - 3.50 (m, H-2’), 3.39 - 3.40 (m, H-4’), 3.46 - 3.47 (m, H-2’, H-5’), 2.26 (s, H-7), 1.53 (s, H-9, H-10); 13C NMR (CD3OD, 150 MHz): δC = 155.5 (C-2), 148.6 (C-4), 129.7 (C-6), 125.4 (C-1), 118.0 (C-5), 111.5 (C-3), 101.3 (C-1’), 76.8 (C-5’), 76.7 (C-3’), 73.6 (C-2’), 71.6 (C-8), 70.0 (C-4’), 61,4 (C-6’), 30.5 (C-10), 30.4 (C-9), 14.7 (C-7).
Secopenthaclethroside (3): White amorphous powder; 1H NMR (DMSO-d6, 600 MHz): δH = 5.93 (d, J = 4.3 Hz, H-6), 4.28 (d, J = 7.9, H-1’), 3.97 (t, J = 7.0 Hz, H-17β), 3.67 (d, J = 12.4 Hz, H-7β), 3.60 - 3.64 (m, H-6’), 3.57 (t, J = 7.4 Hz, H-17α), 3.41 (d, J = 4.0 Hz, H-7α), 3.39 - 3.40 (m, H-6’), 3.15 (t, J = 8.9 Hz, H-3’), 3.09 - 3.13 (m, H-5’), 3.05 (t, J = 8.8 Hz, H-4’), 2.95 (td, J = 8.6, 4.3, H-2’), 2.51 - 2.53 (m, H-10), 2.16 (d, J = 4.2 Hz, H-5), 2.11 (brs, H-13), 1.92 - 1.93 (m, H-14), 1.90 (d, J = 3.7, H-3), 1.78 - 1.82 (m, H-11), 1.65 - 1.67 (m, H-1), 1.49 (o, H-12), 1.47 (o, H-15), 1.39 - 1.42 (m, H-2), 1.23 - 1.25 (m, H-9), 1.22 (s, H-18), 1.12 (s, H-20); 13C NMR (DMSO-d6, 150 MHz): δC = 178.2 (C-19), 103.0 (C-6), 97.3 (C-1’), 89.8 (C-16), 77.3 (C-3’), 77.1 (C-5’), 73.8 (C-2’), 73.7 (C-7), 70.4 (C-4’), 61.9 (C-17), 61.4 (C-6’), 56.8 (C-9), 56.1 (C-5), 49.8 (C-8), 45.6 (C-15), 44.1 (C-4), 42.0 (C-13), 39.2 (C-10), 37.9 (C-14), 36.6 (C-1), 30.4 (C-3), 26.9 (C-18), 26.0 (C-12), 19.6 (C-20), 18.6 (C-11), 18.0 (C-2).
Caffeoylputrescine (4): Yellow amorphous powder; 1H NMR (CD3OD, 500 MHz): δH = 7.41 (d, 15.7 Hz, H-7’), 7.02 (d, J = 2.0, H-2’), 6.90 (dd, J = 8.3, 2.0 Hz, H-6’), 6.78 (d, J = 8.3 Hz, H-5’), 6.40 (d, 15.7 Hz, H-8’), 3.30 - 3.35 (m, H-2), 2.95 - 2.99 (m, 2H, H-5), 1.67 - 1.74 (m, H-4), 1.62 - 1.67 (m, H-3); 13C NMR (CD3OD, 125 MHz): δC = 169.6 (C-9’), 149.8 (C-4’), 146.9 (C-3’), 142.5 (C-7’), 128.3 (C-1’), 122.8 (C-6’), 118.4 (C-8’), 116.6 (C-5’), 115.1 (C-2’), 40.6 (C-5), 39.6 (C-2), 27.6 (C-3), 26.0 (C-4).
β-sitosterol 3-O-β-D-glucopyranoside (5): White crystals from methanol, mp: 290˚C - 292˚C [(lit. 290˚C - 291˚C] [30].
2-hydroxymethyl-5-(2-hydroxypropan-2-yl)phenol (6): Yellow oil; 1H NMR (CD3OD, 500 MHz): δH = 7.19 (d, 7.8, 1H, H-3), 6.94 (d, J = 1.8, H-6), 6.91 (dd, J = 7.8, 1.8, H-4), 4.52 (s, H-7a/7b), 1.50 (s, H-9/10); 13C NMR (CD3OD, 125 MHz): δC = 156.1 (C-1), 151.7 (C-5), 129.1 (C-3), 126.6 (C-2), 116.7 (C-4), 112.7 (C-6), 72.9 (C-8), 61.1 (C-7), 32.0 (C-9/10).
Sucrose 7: White amorphous powder; 1H NMR (CD3OD, 600 MHz): δH = 5.40 (d, J = 4.0 Hz, H-1), 4.11 (d, J = 8.3 Hz, H-3’), 4.04 (t, J = 5.02 Hz, H-4'), 3.80 - 3.84 (m, H-4), 3.79 - 381 (m, H-6), 3.76 - 3.79 (m, H-5’), 3.77 - 3.78 (m, H-6’), 3.73 - 3.75 (m, H-6), 3.69 - 3.74 (m, H-3), 3.60 - 3.65 (m, H-1’), 3.42 - 3.45 (m, H-2), 3.37 (dd, J = 5.0; 1.8 Hz, H-5); 13C NMR (CD3OD, 150 MHz): δC = 103.9 (C-2’), 92.2 (C-1), 82.3 (C-5’), 77.8 (C-3’), 74.2 (C-4’), 73.2 (C-3), 72.8 (C-4), 71.8 (C-2), 69.8 (C-5), 62.5 (C-1’), 61.9 (C-6’), 60.7 (C-6).
7. Conclusion
In the present study, the seeds of the medicinal plant P. macrophylla were investigated, leading to the isolation of a mixture of two new aromatic monoterpene glycosides, pentamacrophylloside A (1a) and pentamacrophylloside B (1b), together with six known compounds. Their structures were elucidated mainly by extensive spectroscopic analysis, high-resolution mass spectrometry and by comparison of their spectral data with those of related compounds. The chemophenetic significance of their isolation was discussed. To the best of our knowledge, this is the first report on the isolation of aromatic monoterpenoids from a plant of the genus Pentaclethra.
Acknowledgements
The authors are grateful to the Alexander von Humboldt Foundation (AvH), Bonn, Germany for the financial support of this work. We thank the Rhineland Palatinate Centre of Natural Products Research (Mainz, Germany) for funding part of the analytical chemistry involved. We would also thank the Yaoundé-Bielefeld Bilateral Graduate School Natural Products with Antiparasitic and Antibacterial Activities (YaBiNaPa) for recording the mass spectra of some of the isolated compounds.