β-Hydrosilylation of Allylic Derivatives with Cyclic and Linear Siloxanes in the Presence of Platinum Catalysts ()

1. Introduction
An important and well-known class of polymers is represented by silicones [1] [2] [3]. Several methods are known to produce organo functional silanes. One of the most frequently used is the transition metal catalysed hydrosilylation of olefins bearing functional groups [4] [5] [6] [7]. Hydrosilylation reactions are generally carried out in the presence of platinum catalyst [8].
Very few siloxanes resulting from a selective β-addition of
and linear hydrosilylating agent to the double bond of terminal olefins functionalized in the allylic position have been prepared to date. One interesting example is the quantitative β-hydrosilylation of the allyl alcohol with
described by Zhang and Laine [9]. In the other cases, characterization of reactions products seemed to be difficult [9].
We reported previously [10], the hydrosilylation conditions to obtain the product β and minimized the by-products (isomerization, hydrogenation) of this reaction, it was found that the use of 1 × 10−5 mole cat./mole olefin for the Karstedt catalyst or 1 × 10−3 mole cat./mole olefin for the platinum black at 40˚C allows the reaction to be carried out in a highly regioselective manner (100% of the β isomer).
Igarashi and al. [11], studied the hydrosilylation reactions of allyl derivatives with hydrosilanes (2,4,6,8-tetramethyl-cyclotetrasiloxane
). These were carried out in the presence of the Karstedt’s catalyst in order to compare their structure-reactivity relationship, but the reaction was in selective with low yield (between 50% - 70%).
In this paper, we wish to report our results on the hydrosilylation of the substituted allylbenzene with
and linears hydrosilylating agents, 1,1-3,3-tetramethyl-disiloxane and MDHM in the presence of platinum catalysts. The choice of platinum catalysts used in this research for the hydrosilylation reaction is platinum (0)-1,3-divinyl-1,1,3,3-tetramethyldisiloxane complex solution or also known as Karstedt’s catalyst and platinum black. Also, the influence of substitution of allyl benzyl derivatives at different positions, amount of catalyst and reaction temperature on regioselectivity was studied.
2. Results and Discussion
Initially, the substituted allylbenzenes 1 (1.1 equiv.) were reacted with
(1.0 equiv.), using 1 × 10−5 mole of Karstedt’s catalyst/mole olefin or 1 × 10−3 mole of platinum black/mole of olefin in dry toluene at temperatures 40˚C and 90˚C for 3 hours, a reaction leading to the hydrosilylated products 3 and 4 took place. These latter could be isolated in excellent yields and good purity just by bulb to bulb elimination under high vacuum at 100˚C of the isomerized olefins (usually 3% to 11%) and traces (≤1%) of the reduced compound. Neither the 2160 cm−1 Si-H infrared band norits 1H NMR peak at 4.61 ppm was detectable in the finalcrude product. Results are reported in Table 1.
The results indicate that the Karsted’ catalyst shows excellent catalytic performance for the hydrosilylation of Allylbenzene derivatives. The conversion of Allylbenzene reaches up to 96% with a higher than 99% β-adduct selectivity (entry 1, Table 1). Meanwhile, 94% conversion of Allylbenzene with 98.3% selectivity for the β-adduct was obtained under the similar conditions when platinum black was used (entry 2, Table 1).
![]()
Table 1. Hydrosilylation of alkenes catalyzed by Pt.
Reaction condition: allylbenzene 18.2 mmol,
4.13 mmol, Karstedt 10−5 mol of Pt/mole of olefin and 1 × 10−3 mole of Pt/mole of olefin for platinum black. aThe selectivity of β-adduct, the byproduct are α-adduct and alkane.
On the other hand, the conversion of 1-allyl-4-methylbenzene is increased to 98% when 1 × 10−5 mole of Karstedt’s catalyst/mole olefin was used. The conversion of 1-allyl-4-(trifluoromethyl)benzene reaches up to 50% and 46% with 96.1% and 95.8% β-adduct selectivity with Karstedt’s catalyst and platinum black respectively (entry 12, Table 1). The reactivity of this olefin is low for platinum catalyst due to the steric effect.
2.1. Effect of Amount of Pt on Hydrosilylation of Allylbenzene
The effect of the amount of catalyst on the conversion of allylbenzene is illustrated in Table 2. It can be seen that higher conversion is obtained when was used low quantity of catalyst during the same reaction time. 85% conversion with 99.7% selectivity of β-adduct is obtained by using 3 × 10−5 mol catalyst/mol of olefin, however, the better selectivity is attained by using lower amount of catalyst (i.e. 10−5 mol Karstedt and 10−3 mol Platinum Black catalysts/mol of olefin). The more catalyst leaded to generating propylebenzene through hydrogenation of allylbenzene. Similar results were found when X. Yang and co-workers used
![]()
Table 2. Effect of amount of Pt-catalyst on hydrosilylation of Allylbenzene.
Reaction condition: allylbenzene 18.2 mmol,
4.13 mmol, 40˚C, 3 h.
iron as a catalyst [12].
2.2. Effect of Reaction Temperature on the Hydrosilylation
The effect of temperature on catalytic activity and selectivity of the hydrosilylation reaction is shown in Figure 1 (using Speier’s catalyst as an example). With the increase of temperature, the time needed for 100% styrene conversion (t100) is shortened, while the selectivity for the β-adduct is kept at around 100%. This indicates that the temperature promotes the catalytic activity but has only a slight influence on the β-adduct selectivity (Figure 1(b)).
Indeed, the effect of reaction temperature on the catalytic activity was investigated and the results are illustrated in Table 3. It indicates that the activity of the Karstedt and platinum black is higher at 40˚C. Using the same amount of catalyst, the conversion of the substrate is between 71% and 79% at 90˚C after 3 h. When the temperature is 40˚C, the conversion of Allylbenzene is between 94 and 96% within 3 h.
2.3. Hydrosilylation of Allylbenzene with Various Silanes
Under similar conditions, different silanes were used (Table 4). The conversion of Allylbenzene is 96%, 94% and 90%, 87% (Karstedt’, platinum black) when
and MDHM were used, respectively. The corresponding selectivity of β-adduct is 100%. Tetramethyl disiloxane gave the conversion 72% and 71% with high selectivity of β-adduct, is 100%.
![]()
Figure 1. Effect of temperature on the hydrosilylation reaction of allylbenzene: (a) catalytic activity and (b) selectivity to β-adduct.
![]()
Table 3. Effect of temperature on the hydrosilylaiton of Allylbenzene with
.
Reaction condition: allylbenzene 18.2 mmol,
4.13 mmol. (0.3 μl of 10−5 mol of Pt/mole of olefin for Karstedt’s catalyst) and (0.244 g, 1 × 10−3 mole of Pt/mole of olefin for platinum black), 3 h.
![]()
Table 4. Scope of silane for Pt catalyzed hydrosilylation of Allylbenzene.
Reaction condition: allylbenzene 18.2 mmol,
4.13 mmol. For Karstedt (0.3 μl, 10−5 mol of cat./mole of olefin) and (0.244 g, 1 × 10−3 mole of Pt/mole of olefin) for platinum black, 40˚C, 3 h.
3. Conclusion
In summary, we have developed a simple and easy method to synthesize functionalized siloxanes by
, MDHM and 1,1-3,3-tetramethyldisiloxane with a high regioselectivity. Karstedt’s-catalyst appeared to be most efficient. The same kind of reactivity and selectivity was observed with black platinum, although 100 times more catalyst was used. The nature of substituents on the aromatic ring or the hydrosilylating agent has a little influence on the β/α ratio. However, the amount of catalyst and reaction temperature appeared to be more important.
4. Experimental Method
All chemical reagents were commercially available. Toluene was distilled from sodium benzophenone ketyl immediately prior to use, platinum (0)-1,3-divinyl-1,1,3,3-tetramethyldisiloxane complex solution (Karstedt’s catalyst, Pt ~ 2% in xylene), 2,4,6,8-tetramethylcyclotetrasiloxane (
≥99.5% and ≥99.999% trace metals analysis). All other chemicals were used as received without further purification. All manipulations involving air-sensitive species were carried out in Schlenk apparatus under dry nitrogen. lH.n.m.r, 13C and 29Si spectra were recorded on a Brucker type device 500, 125 and 99 MHz instrument with CDCl3 as the solvent; the chemical shifts were reported in ppm, by reference to Me4Si. the coupling constants (J) are expressed in Hertz (Hz).
5. Experimental Procedures
a) Preparation of 1,3,5,7-tetra-(3-(phenyl)propyl)-1,3,5,7-tetramethyl-cyclotetrasiloxane (3a) from allylbenzene with
in the presence of Karstedt’s catalyst is representative.
The Solution of allylbenzene (2.4 ml, 18.2 mmol) and Karstedt’s catalyst (0.3 μl of a 0.336 mol/l solution of Pt/mole of olefin) in toluene (5 ml) was warmed to 40˚C under argon.
(1 ml, 4.13 mmol) was added slowly and subsequently heated at To this stirred solution wich was subsequently heated at 40˚C for 3 h. the hydrosilylation was followed by 1H NMR and the reaction stopped once the Si-H signal has disappeared. The solvent was then evaporated under reduced pressure. Volatile by-products and excess of olefin were removed by bulb to bulb distillation (100˚C, 0.01 torr). 1,3,5,7-tetra-(3-(phenyl)propyl)-1,3,5,7-tetramethyl-cyclotetrasiloxane was obtained as viscous oil in a 96% yields.
b) Preparation of 1,3,5,7-tetra-(3-(phenyl)propyl)-1,3,5,7-tetramethyl-cyclotetrasiloxane (3a) from allylbenzene with
in the presence of platinum black is representative.
A solution of allylbenzene (2.4 ml, 18.2 mmol) and platinum black (0.244 g, 1 × 10−3 mole of Pt/mole of olefin) in toluene (5 ml) was warmed to 40˚C. To this stirred solution,
(1 ml, 4.13 mmol) was added slowly and subsequently heated at 40˚C for 3 h. After filtration over glass wool, the solvent was evaporated under reduced pressure; volatile by-products and excess of olefin were removed by bulb to bulb distillation (100˚C, 0.01 torr).
c) Preparation of 1,3-di-(3-(phenyl)propyl)-1,1,3,3-tetramethyl-disiloxane (3h) from allylbenzene with 1,1,3,3-tetramethyldisiloxane in the presence of Karstedt’s catalyst is representative.
The Solution of allylbenzene (1.2 ml, 9.1 mmol) and Karstedt’s catalyst (0.3 μl of a 0.336 mol/l solution of Pt/mole of olefin) in toluene (5 ml) was warmed to 40˚C under argon. 1,1,3,3-tetramethyldisiloxane (0.72 ml, 4.13 mmol) was added slowly and subsequently heated at To this stirred solution which was subsequently heated at 40˚C for 3 h. the hydrosilylation was followed by 1H NMR and the reaction stopped once the Si-H signal has disappeared. The solvent was then evaporated under reduced pressure. Volatile by-products and excess of olefin were removed by bulb to bulb distillation (100˚C, 0.01 torr). 1,3-di-(3-(phenyl)propyl)-1,1,3,3-tetramethyl-disiloxane was obtained as viscous oil in a 72% yields.
d) Preparation of 1,3-di-(3-(phenyl)propyl)-1,1,3,3-tetramethyl-disiloxane (3h) from allylbenzene with 1,1,3,3-tetramethyldisiloxane in the presence of platinum black is representative.
A solution of allylbenzene (1.2 ml, 9.1 mmol) and platinum black (0.244 g, 1 × 10−3 mole of Pt/mole of olefin) in toluene (5 ml) was warmed to 40˚C. To this stirred solution, 1,1,3,3-tetramethyldisiloxane (0.72 ml, 4.13 mmol) was added slowly and subsequently heated at 40˚C for 3 h. After filtration over glass wool, the solvent was evaporated under reduced pressure; volatile by-products and excess of olefin were removed by bulb to bulb distillation (100˚C, 0.01 torr).
e) Preparation of 1,1,1,3,5,5,5-heptamethyl-3-(3-phenylpropyl)trisiloxane (3i) from allylbenzene with MDHM in the presence of Karstedt’s catalyst is representative.
The solution of allylbenzene (1.2 ml, 9.1 mmol) and Karstedt’s catalyst (0.3 μl of a 0.336 mol/l solution of Pt/mole of olefin) in toluene (2.5 ml) was warmed to 40˚C under argon. To this stirred solution MDHM (3 ml, 10.92 mmol) was added slowly and subsequently heated at 40˚C for 2 h. The end of the hydrosilylation reactions was determined by the disappearance of the Si-H chemical shift in the 1H NMR spectrum. The solvent and volatile by-products were removed under reduced pressure. Further the product was purified by distillation (70˚C, 0.05 torr) and recovered as a colorless oil in a 90% yield.
f) Preparation of 1,1,1,3,5,5,5-heptamethyl-3-(3-phenylpropyl)trisiloxane (3i) from allylbenzene with MDHM in the presence of platinum black is representative.
A solution of allylbenzene (1.2 ml, 9.1 mmol) and platinum black (0.244 g, 1 × 10−3 mole of Pt/mole of olefin) in toluene (2.5 ml) were warmed up to 40˚C under argon. To this stirred solution MDHM (3 ml, 10.92 mmol) was added slowly and subsequently heated at 40˚C for 2 h. After filtration over glass wool, the solvent and volatile by-products were removed under reduced pressure. The product was purified by distillation and recovered as a colorless oil.
(3a) yield = 96% for Karstedt, 94% for Platinum black. 1H NMR (500 MHz, CDCl3): δ 0.09 - 0.14 (m, 12H, Si-CH3), 0.60 (t, J = 11.46 Hz, 8H, Si-CH2), 1.65 - 1.76 (m, 8H, Si-CH2-CH2), 2.60 - 2.70 (m, 8H, CH2-Ph), 7.19 - 7.36 (m, 20H, CH-Ar); 13C NMR (125 MHz, CDCl3): δ −0.52 (Si-CH3), 17.03 (Si-CH2), 25.15 (Si-CH2-CH2), 39.40 (CH2-Ph), 125.73, 128.33, 128.56 (CH-Ar), 142.66 (C-Ar); 29Si NMR: δ −20.53, −20.46, −20.43; HRMS Calcd for (C40H56O4Si4)[M+]: 709.3256, Found: 709.3278.

(3b) yield = 94% for Karstedt, 88% for Platinum black. 1H NMR (500 MHz, CDCl3): δ0.01 (s, 12H, Si-CH3), 0.55 - 0.61 (t, J = 3.5 Hz, 8H, Si-CH2), 1.41 - 1.59 (m, 8H, Si-CH2-CH2), 2.26 (s, 12H, CH3-Ph), 2.51 - 2.59 (m, 8H, CH2-Ph), 7.00 - 7.22 (m, 16H, CH-Ar); 13C NMR (125 MHz, CDCl3): δ −0.64 (Si-CH3), 17.88 (Si-CH2), 19.89 (CH3-Ph), 24.39 (Si-CH2-CH2), 37.41 (CH2-Ph), 126.44, 129.52, 130.73 (CH-Ar), 136.46, 141.45 (C-Ar); 29Si NMR: δ −20.12, −20.08; HRMS Calcd for (C44H64O4Si4)) [M+]: 769.3241, Found: 769.3245.

(3c) yield = 95% for Karstedt, 89% for Platinum black. 1H NMR (500 MHz, CDCl3): δ 0.04 (s, 12H, Si-CH3), 0.61 - 0.68 (t, J = 13.5 Hz, 8H, Si-CH2), 1.42 - 1.53 (s, 8H, Si-CH2-CH2), 2.30 (s, 12H, CH3-Ph), 2.59 - 2.68 (m, 8H, CH2-Ph), 7.01 - 7.46 (m, 16H, CH-Ar); 13C NMR (125 MHz, CDCl3): δ −0.01 (Si-CH3), 18.03 (Si-CH2), 19.72 (CH3-Ph), 22.12 (Si-CH2-CH2), 34.63 (CH2-Ph), 126.15, 127.55, 128.92 (CH-Ar), 131.70, 137.53 (C-Ar); 29Si NMR: δ −20.11, −20.06; HRMS Calcd for (C44H64O4Si4)) [M+]: 769.3242, Found: 769.3248.
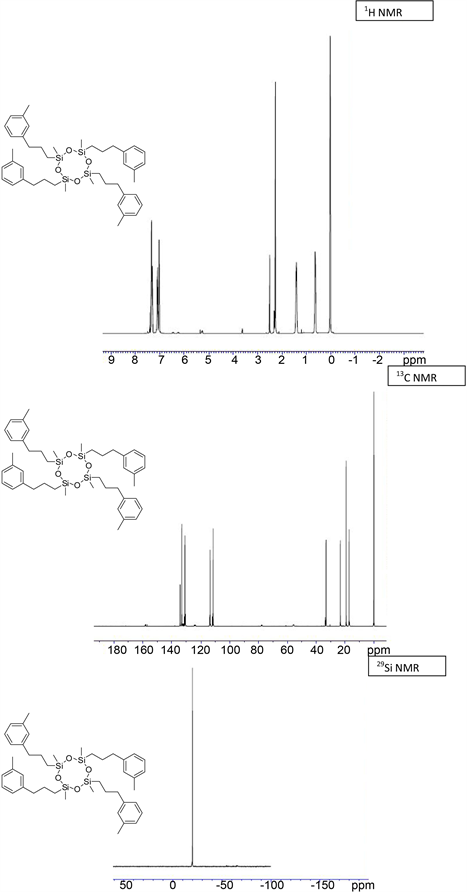
(3d) yield = 98% for Karstedt, 85% for Platinum black. 1H NMR (500 MHz, CDCl3): δ 0.01 (s, 12H, Si-CH3), 0.62 - 0.69 (t, J = 10 Hz, 8H, Si-CH2), 1.40 - 1.49 (m, 8H, Si-CH2-CH2), 2.54 (m, 12H, CH3-Ph), 2.60 (m, 8H, CH2-Ph), 7.00 - 7.12 (m, 16H, CH-Ar); 13C NMR (125 MHz, CDCl3): δ −0.00 (Si-CH3), 18.01 (Si-CH2), 19.61 (CH3-Ph), 22.08 (Si-CH2-CH2), 34.12 (CH2-Ph), 127.36, 127.98, 128.13 (CH-Ar), 136.65, 136.74 (C-Ar); 29Si NMR: δ −21.87; HRMS Calcd for (C44H64O4Si4)) [M+]: 769.3246, Found: 769.3253.
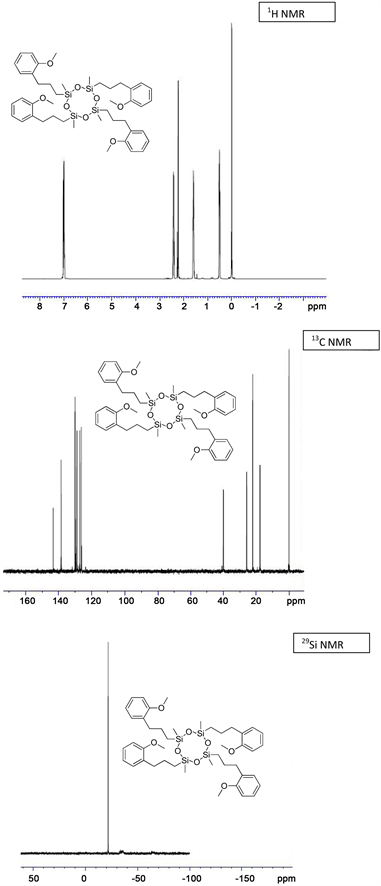
(3e) yield = 53% for Karstedt, 49% for Platinum black. 1H NMR (500 MHz, CDCl3): δ 0.02 (s, 12H, Si-CH3), 0.51 (t, J = 5 Hz, 8H, Si-CH2), 1.52 (s, 8H, Si-CH2-CH2), 2.22 - 2.31 (m, 8H, CH2-Ph), 2.45 - 2.56 (s, 12H, CH3-O), 7.02 - 7.22 (m, 16H, CH-Ar); 13C NMR (125 MHz, CDCl3): δ −0.00 (Si-CH3), 17.61 (Si-CH2), 22.05 (Si-CH2-CH2), 25.65 (CH2-Ph), 39.94 (CH3-O), 116.30, 125.93, 128.75, 129.92 (CH-Ar), 138.48, 143.17 (C-Ar); 29Si NMR: δ −20.87; HRMS Calcd for (C44H64O8Si4)) [M+]: 833.3266, Found: 833.3278.
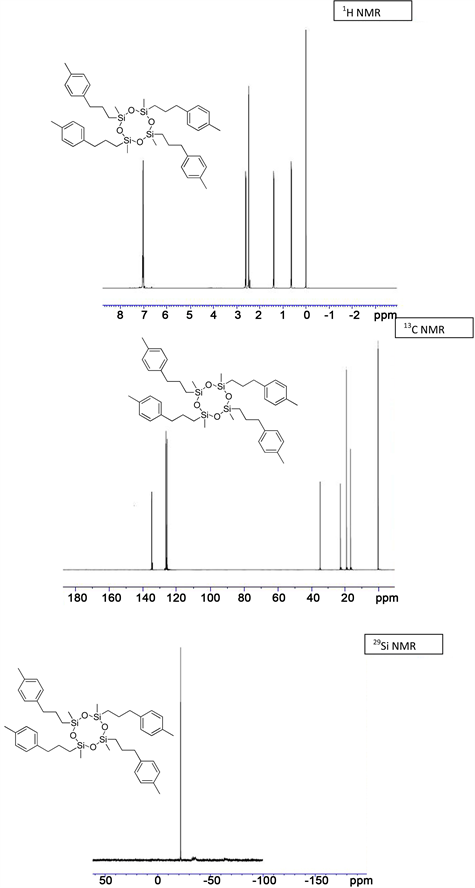
(3f) yield = 50% for Karstedt, 46% for Platinum black. 1H NMR (500 MHz, CDCl3): δ 0.12 (s, 12H, Si-CH3), 0.73 (t, J = 4.5 Hz, 8H, Si-CH2), 1.70 (m, 8H, Si-CH2-CH2), 2.59 - 2.61 (m, 8H, CH2-Ph), 7.15 - 7.38 (m, 16H, CH-Ar); 13C NMR (125 MHz, CDCl3): δ −0.61 (Si-CH3), 19.81 (Si-CH2), 23.46 (Si-CH2-CH2), 28.36 (CH2-Ph), 115.54 (C-F3), 125.30, 128.48 (CH-Ar), 136.33, 144.16 (C-Ar); 29Si NMR: δ −22.06; HRMS Calcd for (C44H52F12O4Si4)) [M+]: 985.2032, Found: 985.2045.
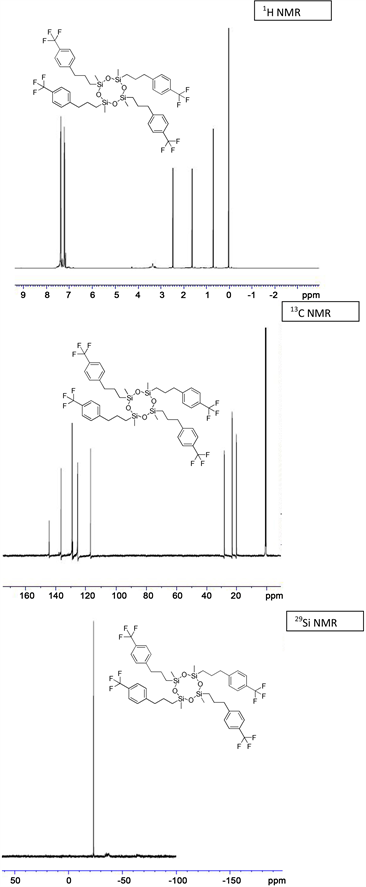
(3g) yield = 52% for Karstedt, 47% for Platinum black. 1H NMR (500 MHz, CDCl3): δ 0.14 (s, 12H, Si-CH3), 0.59 (t, J = 2.5 Hz, 8H, Si-CH2), 1.65 (s, 8H, Si-CH2-CH2), 2.58 - 2.63 (m, 8H, CH2-Ph), 7.05 - 7.51 (m, 16H, CH-Ar); 13C NMR (125 MHz, CDCl3): δ −0.68 (Si-CH3), 19.72 (Si-CH2), 23.61 (Si-CH2-CH2), 28.80 (CH2-Ph), 126.82 (C-Br), 127.01, 129.46, 129.60 (CH-Ar), 131.82, 134.68 (C-Ar); 29Si NMR: δ −23.29, −24.64; HRMS Calcd for (C40H52Br4O4Si4)) [M+]: 1028.8021, Found: 1028.8067.
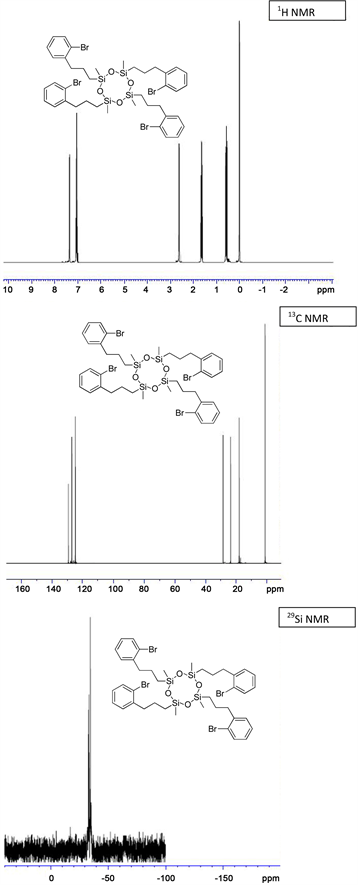
(3h) yield = 72% for Karstedt, 71% for Platinum black. 1H NMR (500 MHz, CDCl3): δ 0.00 (s, 6H, Si-CH3), 0.61 (t, J = 9.41 Hz, 4H, Si-CH2), 1.58 (m, 4H, Si-CH2-CH2), 2.61 (m, 4H, CH2-Ph), 7.01 - 7.39 (m, 10H, CH-Ar); 13C NMR (125 MHz, CDCl3): δ −0.22 (Si-CH3), 20.75 (Si-CH2), 22.02 (Si-CH2-CH2), 29.66 (CH2-Ph), 125.73, 128.33, 128.56 (CH-Ar), 142.66 (C-Ar); 29Si NMR: δ −22.87; HRMS Calcd for (C22H34OSi2)) [M+]: 370.6878, Found: 370.6889.
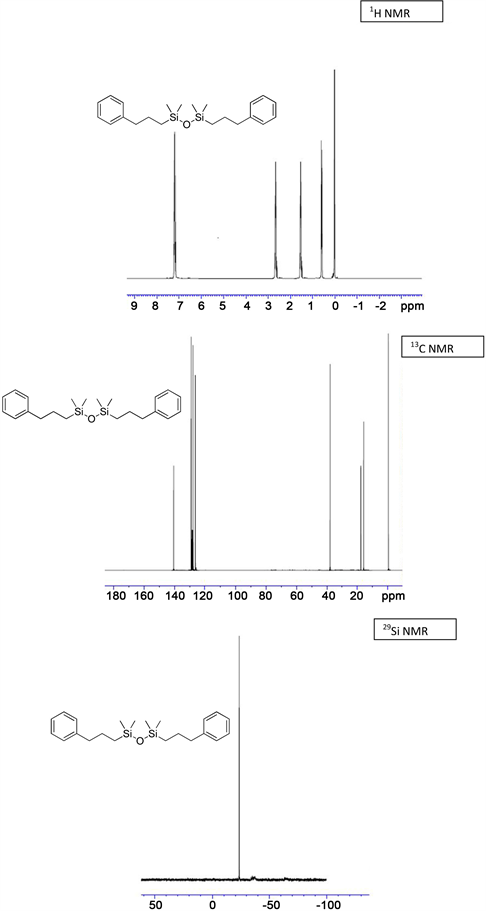
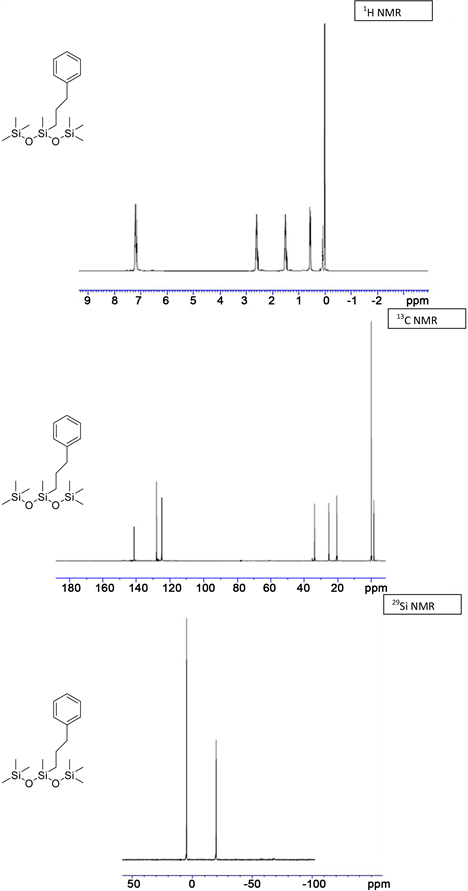
(3i) yield = 90% for Karstedt, 87% for Platinum black. 1H NMR (500 MHz, CDCl3): δ 0.01 (m, 18H, Si-(CH3)3), 0.14 (s, 3H, Si-CH3), 0.61 (t, J = 10.46 Hz, 2H, Si-CH2), 1.58 (m, 2H, Si-CH2-CH2), 2.60 - 2.70 (m, 2H, CH2-Ph), 7.20 - 7.26 (m, 5H, CH-Ar); 13C NMR (125 MHz, CDCl3): δ −3.77 (Si-CH3), 0.33 (Si-(CH3)3), 20.83 (Si-CH2), 25.46 (Si-CH2-CH2), 35.66 (CH2-Ph), 125.98, 128.39, 128.44 (CH-Ar), 142.52 (C-Ar); 29Si NMR: δ −21.19 (Si-CH3), 6.81 (Si-(CH3)3); HRMS Calcd for (C16H32O2Si3) [M+]: 340.6858, Found: 340.5423.