Titrimetric and Spectrophotometric Determination of Some Thiadiazole Derivatives by Using Amplification Reactions ()
1. Introduction
The importance of thiadiazoles was improved when the sulfa-drugs were invented [1]. These compounds are characterized by high biological activity and found application as antifungal and antimicrobial compounds as well as antitumer compounds [2] [3] [4]. The coupling of acetylene with thiadiazoles showed anti-choline esterase enzyme. They were also incorporated in the thermal moldable polymers [5]. Some derivatives are employed as dyes, ion exchange resins in which thiadiazoles represent a major part were efficiently applied in the removal of several cations with 100% efficiency in some instances [6]. The 1,3,4-thiadiazole derivatives were preferably used as additives for the extreme pressure greases because they are biodegradable and hence friendly for the environment [7]. The interest in thiadiazole derivatives is growing and many new compounds are continuously synthesized for antifungal purposes (Karaburun, et al. [8] ). Mahmood et al., synthesized and evaluated the activity of some thiadiazole Schiff base derivatives as antibacterial agents [9]. Compounds belonging to the group of 5-substituted 4-(1,3,4-thiadiazol-2-yl) benzene-1,3-diols exhibited a broad spectrum of biological activity, including antibacterial, antifungal, and anticancer properties. Chudzik et al. [10], reported the activity of 4-(5-methyl-1,3,4-thiadiazole-2-yl) benzene-1,3-diol, against pathogenic fungi with the lowest toxicity to human cells. The thiadiazole ring exhibits several specific properties: it is a bioisostere of pyrimidine or benzene rings with prevalence in biologically active compounds; the sulfur atom increases lipophilicity and combined with the mesoionic character of thiadiazoles imparts good oral absorption and good cell permeability, resulting in good bioavailability. The 2-amino-1,3,4-thiadiazole derivatives exhibited antileishmanial activity [11]. Kaur and Sing [12] published a review on the Thiadiazole analogs as potential pharmacological agents. Thiadiazole derivatives are utilized therapeutically for various diseases thanks to their antiviral [13], anticonvulsant [14] anti-inflammatory [15], anticancer [16] activities. It was also reported that thiadiazole derivatives demonstrated antithyroid activities [17]. Thiadiazole derivatives and its metal compounds have antibacterial, antifungal, antitumoral, antiproliferative and antioxidant properties. The effects caused by thiadiazole ligand and its metal complexes upon the fatty acids and lipophilic vitamins in livers of rats were examined by Parlak et al. [18]. Many methods were employed for the determination of 1,3,4-thiadiazoles. High performance liquid chromatography, HPLC, was used in the analysis of rice for traces of thiadiazoles after extraction and photometric detection at 313 nm. Hitachi Gel was used as the stationary phase for the separation and refractive index for the detection of these compounds in the thermal decomposition products of thiobenzamide [19]. Amplification reactions are divided into direct and indirect methods [20] [21]. The indirect ones found many applications in the determination of various organic compounds [22], where they first react with iodine and the resultant iodide is then involved in an amplification reaction. Recently, the method was applied for the determination of some thiosemicarbazone derivatives according to the equation:
(1)
The reactions are accompanied by a drop in the pH of the reaction mixture indicating the formation of the acids [23]. The present paper is concerned with the utilization of amplification reactions in the determination of some 1,3,4-thiadiazoles, compounds namely: 2-amino-5-mercapto-1,3,4-thiadiazole (I); 2,5-dimercapto-1,3,4-thiadiaole (II) and 2,5-diamino-1,3,4-thiadiazole (III) and for 2,5-dihydrazino-1,3,4-thiadiazole (IV), 5-(((5-nitrofuran-2-yl)methylene) amino)-1,3,4-thiadiazole-2-thiol (V) and 5-mercapto-2[(3[5’-nitro-2-furyl]-prop-2-enylidene)amino]-1,3,4-thiadiazole (VI). The evaluation was done by titration with thiosulfate standard solution and spectrophotometrically by measuring the absorbance of the blue starch-iodine complex.
2. Experimental
Apparatus: the spectral measurements were carried out on a U 2000 spectrophotometer supplied from Hitachi. The IR spectra were recorded on a Pye-Unicam SP 3-100 IR spectrophotometer.
Chemicals: All of the chemicals and reagents were of analytical grade. Distilled de-ionized water was used for rinsing and preparation of working solutions. The following thiadiazole compounds were prepared according to the published methods [24] [25] [26] [27] [28] and determined:
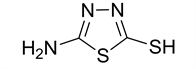
5-amino-1,3,4-thiadiazole-2-thiol (1)
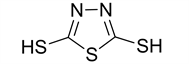
1,3,4-thiadiazole-2,5-dithiol (2)
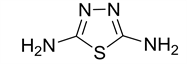
1,3,4-thiadiazole-2,5-diamine (3)
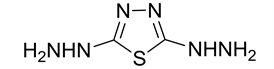
2,5-dihydrazinyl-1,3,4-thiadiazole (4)
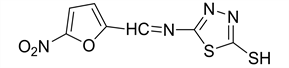
5-(((5-nitrofuran-2-yl) methylene)amino)-1,3,4-thiadiazole-2-thiol (5)
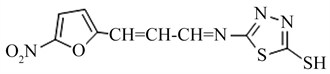
5-mercapto-2-[(3[5’-nitro-2’-furyl]-prop-2’-enylidene)amino]-1,3,4-thiadiazole (5)
Working solutions:
1) Thiadiazole solutions: accurately about 0.1 g of each compound was dissolved in 6 mL of ethanol (99%) and water was added up to a volume of 100-mL in a calibrated flask.
2) Potassium iodate solution 0.01 N; sodium thiosulfate 0.01 N; potassium iodide 0.5%; iodine 0.12% in chloroform and bromine water solution was prepared by gradually diluting 5-mL of bromine with water up to a final volume of 250 mL.
3) Buffers: a series of acetate buffer solutions were prepared according to the standard methods [19].
4) Interfering solutions: The interference solutions were prepared to be 10,000 µg·mL−1 in the appropriate solvents.
Procedure: 1.0 mL aliquot of thiadiazole solution was placed in a 100-mL separating funnel and 10 mLs of acetate buffer solution and 10 mLs of iodine solution were added. After 5 mins of shaking, the water phase was separated from the upper organic phase. The water layer was washed twice with 10-ml aliquots of chloroform to remove residual iodine. Bromine water was added to the aqueous phase and the mixture was shaken for 2 mins. Excess bromine was removed by adding 2 mLs of formic acid. Finally 0.5 g of potassium iodide were added. The generated iodine was determined by titration with standard thiosulfate solution.
For spectral detection thiadiazole solution aliquot containing 50 µg was found enough and the same procedure was followed with the exception of addition of KI where 2-mLs of 0.5% KI solution followed by 1 ml of starch indicator solution. The absorbance of the resultant solutions was then measured. The effects of various operating parameters on the response were studied to reach the optimum conditions of the analysis.
3. Results and Discussion
Verification of the composition of thiadiazole compounds:
The compounds were prepared according to the published methods and melting points, UV and IR spectra were recorded. The results are listed in Table 1. The assignment of the IR peaks is presented in Table 2.
![]()
Table 1. Summary of the results verification of the composition of thiadiazole compounds.
![]()
Table 2. The assignment of the IR peaks.
Effect of pH of the solution:
The reactions were carried out in media of various pH values and the response was recorded. For compounds (I - III) the response is plotted in Figure 1. For compounds IV - VI, the results are shown in Figure 2. Numerically the: Effect of medium on the reaction (10 mL reagent) extent of 1.00 mg of compounds (3) is given in Table 3. It is clear that the optimum pH value is dependent on the chemical composition. However, Compounds II, V and VI require more acidic conditions than the others to attain the highest reaction extents. This may be the effect of the thiol group.
Effect of Iodine amount:
After the optimization of the pH of the reaction mixture which allows the best reaction extent of the thiadiazole derivatives with iodine, the effect of Iodine solution volume was studied. Various volumes (1 - 20 mL) of the iodine dissolved in chloroform (0.12%) solution were employed in the reaction. After reaction with thiadiazole, iodine is transferred into the aqueous layer for determination by titration. The best response could be obtained with 5-mLs aliquots of the iodine solution. Thus, such amount was fixed for all experiments.
The reaction of thiadiazoles with iodine was allowed to proceed for various intervals of time and the response was recorded. For compounds I and II five minutes appeared enough to give the highest yield. On the other hand, compound III required only one min while two mins were required for compounds V and VI to complete reaction with iodine. This can be related to the acidity of the hydrogen atom of the functional groups especially the NHNH2, NH2 and SH.
![]()
Figure 1. Effect of pH of the medium on the reaction of compounds 1; 2; and 3.
![]()
Figure 2. Effect of pH of the medium on the reaction of compounds 4, 5, and 6.
![]()
Table 3. Effect of medium on the reaction (10 mL reagent) extent of 1.00 g of compounds (III).
The amount of bromine water necessary to oxidize the iodide produced by the first reaction was studied. It appeared that aliquots of 1 - 4 mLs were adequate for the complete oxidation of iodide into iodate. Thus, 2 mLs of saturated bromine water were added to each sample solution and fixed all over the work. The oxidation can be aided by shaking the reactants for not less than (3 - 5) mins. For all experiments three minutes reaction time was chosen. However, any excess bromine needs to be destroyed in order not to consume part of the thiosulfate solution later on. Table 4 shows the effect of formic (90%) volume required for the destruction reaction. Below 1.0 mLs, the destruction is incomplete and the thiosulfate was consumed in excess. Thus, 2-mL aliquot was fixed for such reaction.
The final step of the amplification process involves the reaction of the iodate ions, produced therein, with potassium iodide. Each iodate ion requires five iodide ions for this reaction. Potassium iodide, therefore, must be added in excess (0.2 - 1.0 g) to the reaction mixture. However, an amount of 0.5 g of solid potassium iodide was found enough to perform this job. The titration was carried out after various time intervals up to ten minutes. The results of compound (I) clearly showed that titration can be done immediately after the addition and mixing of potassium iodide crystals with the reaction mixture, where iodine is liberated.
Calculation of the number of iodine moles per mole of Thiadiazole derivatives:
One mg of compound (I) is equivalent to mLs of standard 0.01 N sodium thiosulfate (0.244 meq) and consequently to 0.244 meq of iodine. The overall amplification factor, therefore, is 0.244/0.0075 = 12. As mentioned above, iodate reacts with five iodide ions to give three iodine molecules, i.e. the iodate is amplified six times. The division of overall amplification on the iodate amplification gave (2). Therefore, each mole of compound (I) releases two iodide moles by reaction with iodine, i.e. one mole of iodine is required by one mole of compound (I). Similarly. It was found that:
one mole of iodine reacts with one mole of II;
one mole of iodine reacts with one mole of III;
three moles of iodine react with one mole of IV;
half a mole of iodine react with one mole of V;
half a mole of iodine reacts with one mole of VI. Therefore:
1.0 mL of 0.01 N thiosulfate solution = 0.108 mg of compound I
1.0 mL of 0.01 N thiosulfate solution = 0.126 mg of compound II
1.0 mL of 0.01 N thiosulfate solution = 0.1 mg of compound III
1.0 mL of 0.01 N thiosulfate solution = 0.041 mg of compound IV
1.0 mL of 0.01 N thiosulfate solution = 0.4 mg of compound V
1.0 mL of 0.01 N thiosulfate solution = 0.435 mg of compound VI
Mechanism of Amplification Reaction:
On basis of experiments and calculations, the following mechanism may be proposed for the amplification reactions of thiadiazole derivatives:
![]()
Table 4. The effect of formic (90%) volume required for the destruction of excess bromine water on the reaction of 1.00 mg of compound (I).
(2)
(3)
(4)
(5)
(6)
(7)
The increase of the acidity of the reaction media supports these mechanisms reaching pH values of (4 - 5) and the occurrence of gas evolution. Further the smell of ammonia was noticed in the case of reaction 3, 5, and 6 and the hydrogen sulfide in the case of reaction (4). The hydroiodic acid or the iodide ions produced undergo the following amplification reactions:
(8)
(9)
(10)
Each iodide ion, therefore, produces three iodine molecules or six iodine atoms and the amplification factor will be (12) for compounds I - III, (36) for compound IV and only (6) for compounds V and VI.
Precision and Accuracy:
Under the optimum conditions, the precision of the method was checked by evaluating the relative standard deviation at three different concentrations of each thiadiazole derivative. The results are listed in Table 5. The low precision is related to the presence of sources of errors mainly due to the measurement of the volume of thiosulfate solution.
Interference:
The effects of some organic and ionic compounds on the reaction of thiadiazoles with iodine were studied. The percentage changes in the response of 1.0 mg of compound I in the presence of the interfering compound at 1, 5, 10, 50, 100, 250 and 500 times of its concentration are shown in Table 6. It appears that, compounds having similar functional groups cause interference effects. The use of thiadiazole in pharmaceutical preparations [23] and as additives in special greases [6], were the reason of studying the effects of the other chemicals shown in Table 6. Glucose and lithium 12-hydroxy stearate have only minor interferences due to the hydroxyl group of the weak acidity. However, neither of sodium chloride, lithium stearate, or base oil showed any interference on the analytical performance. The same conclusion holds for other thiadiazole derivatives.
Spectrophotometric-Amplification Reactions:
Using the same experimental optimum conditions the detection of the blue iodine-starch complex could be accomplished by measuring the absorbance at 605 nm [22]. Before the detection step, potassium iodide was used as a 0.5% solution and several amounts were examined (0.5 - 4.0 mL). An aliquot of 2.0 mL was found adequate for this purpose. The volume of the starch indicator solution was not critical and 1 - 5 mLs gave no differences in the response at 605 nm. However, a volume of 1.0 mL was fixed for all measurements.
The color of the solution develops immediately and no absorbance can be measured directly after the addition of the starch solution. The measurement of the absorbance after various intervals of time indicated that 30 minutes must not be exceeded and color may begin to diminish gradually. Thus, all absorbance measurements were done within the first 20 min after adding starch solution and dilution to the appropriate volume in the volumetric flask. Calibration graphs for the six compounds were constructed. The analytical parameters are calculated and listed in Table 7. The accuracy and precision of the method were tested at three levels of concentration and the results are given in Table 8. It is clear that the recovery values were acceptable and ranges between 95% to 102%. The interference effects were similar to those noticed above for the titrimetric method.
![]()
![]()
Table 5. Relative standard deviation, RSD, recovery and relative error for three levels of concentrations of thiadiazole derivatives*.
*Results are the mean of five readings from which the blank result is subtracted.
![]()
Table 6. Percentage interferences of various chemicals with the analysis of 1.00 mg of compound I using the optimum conditions.
SI = Serious interference.
![]()
Table 7. The analytical parameters of the spectral determination of thiadiazoles.
![]()
Table 8. Accuracy and precision of the spectral determination of thiadiazoles.
4. Conclusion
Thiadiazole derivative can be analyzed efficiently by amplification reactions with iodine with appreciable accuracy and amplification factors ranging from 6 to 36. Spectrophotometric as well as titrimetric detection can be used to evaluate the response of the method. The method is characterized by relatively high sensitivity and precision with acceptable recovery values (94.4% - 102.0%) at several levels of concentration. Compounds with functional groups similar to those of thiadiazoles may interfere in the analysis.