Design, Synthesis, Crystal Structure and Photoluminescence Properties of Four New Europium (III) Complexes with Fluorinated β-Diketone Ligand ()
1. Introduction
The photoluminescence properties of Lanthanide complexes with organic ligands have been greatly enhanced, and led to the development of strong luminescent Lanthanide complexes with important applications in medical, industrial and biological fields [1] - [7]. Europium (III) complex with organic ligands is an example of strong luminescent Lanthanide complex and Europium (III) complexes that have great importance in materials engineering chemistry due to significant improvement in photophysical parameters such as high luminescence emission efficiency, long fluorescence life time, large stokes shift, sharp emission bands [8] [9] [10] [11]. In the past decade various high luminescent europium complexes have been engineered and evaluated for their photoelectronic properties such as OLEDs, electroluminescent displays, bioimaging, sensing and targeting specific DNA structures, melamine detection in milk protein. Europium (III) complexes have also found applications as sensor materials to detect pesticides, temperature, HCl, NO2 gas, HOCl, pH, phosphate, mitochondria and, 8-oxo-dGTP [12] - [20]. Albumin proteins in human serum have also been detected by Europium complexes, which act as sensor materials [21].
Search for novel europium complexes that uses less energy and exhibits desired application such as sensors, OLEDS, etc. is of great importance in photoelectronic materials. Therefore, new europium complexes should have enhanced degree of change in luminescence to be a good sensor material. On the other hand, understanding the relationship between molecular structures and photoelectronic properties of europium (III) complexes gives valuable information in designing future photoelectronic materials with improved properties. It is stated that luminescence of Europium (III) ion originates from forbidden f-f transitions that totally hinder the Europium (III) ion interaction with light. The ligand that forms complexes with Europium (III) ion acts as antenna. This absorbs energy and transfers to Europium (III) ion through intersystem crossing to triplet excited states. In this context, europium complexes with substituted aromatic β-diketones as organic ligands were explored due to efficiency in generating triplet excited states in close contact with europium (III) ion. Thus, various Europium (III) complexes with β-diketones were synthesized and evaluated for their photoluminescent properties [22] [23] [24] [25] [26].
In our previous studies, we synthesized and investigated the molecular structures and photoelectronic properties of octa-coordinate europate (III) complexes using substituted β-diketone ligands [27] [28]. In this study, we want to investigate the molecular structures and photoluminescence properties of four new octa-coordinate Europium (III) complexes 3a, 3b, 3c and 3d, possessing pyridinium, bipyridinium, piperazinium and bipiperidinium as counter cations.
2. Experimental
2.1. Materials and Methods
Reagent grade europium (III) chloride, pyridine, bipyridine, piperazine and bipiperidine were purchased from the TCI chemicals industry, Tokyo and used as such to prepare europium complexes. The ligand 1-phenyl-4,4,4-trifluoromethyl-1,3-butanedione was synthesized in the laboratory. The positive fast atom bombardment (FAB) mass spectrum (MS) of the complexes was obtained on a Nippon Densi JEOL JMS-SX102A spectrometer (JEOL, Tokyo, Japan) using NBA (nitrobenzyl alcohol) as the matrix and DCM (dichloromethane) as the solvent. The instrument was operated in negative ion mode over an m/z range of 100 - 2000. Elemental analysis data were recorded on a Yanako MT-4 analyzer (Yanako Group, Kyoto, Japan). A JASCO V-550 spectrophotometer (JASCO Corporation, Tokyo, Japan) was used for obtaining UV-Vis spectra in dichloromethane with 250 - 900 nm range. HITACHI F-8700 spectrophotometer (Hitachi High-Technologies Corporation, Tokyo, Japan) was used for fluorescence spectra measurements in dichloromethane with 250 - 900 nm range. CCDC No. 1962454, 2047729, 1563207 and 1563206 contain the supplementary crystallographic data for the complexes 3a, 3b, 3c and 3d, respectively.
2.2. General Procedure for the Synthesis of Complexes 3a, 3b, 3c and 3d
In a RB flask, a solution of europium (III) chloride (0.650 g, 0.41 mmol) and 1-phenyl-4,4,4-trifluoromethyl-1,3-butanedione 1 (0.370 g, 1.65 mmol) in absolute ethanol (30 mL) was prepared at room temperature. Under protection from air, slightly excess of pyridine, bipyridine, piperazine, bipiperidine were added to the solution to get complexes 3a, 3b, 3c and 3d respectively. Ethanol was removed by rotary evaporator under reduced pressure. Under protection from air, the residue was repeatedly washed with small portions (5 mL) of warm, dry ethanol. The residual powders were dissolved in ethanol for crystallization. Without protection from air, the crystallized product was filtered off, washed with two portions of cold ethanol, and dried under reduced pressure, affording the complexes 3a, 3b, 3c and 3d as a powder. All four complexes were obtained in moderate to good yields (68% for 3a, 65% for 3b, 75% for 3c and 60% for 3d, respectively).
2.3. Single-Crystal X-Ray Analysis and Structure Determination
Crystals of four compounds 3a, 3b, 3c and 3d were obtained at room temperature by crystallization in DCM-ethanol mixed solvent.
The crystal data were recorded on a Bruker APEX II KY CCD diffractometer equipped with graphite monochromatized Mo-Kα radiation of wavelength 0.71073 Å from a sealed micro focus tube, and a nominal crystal to area detector distance of 58 mm. X-ray generator settings were 50 kV and 30 mA. The data were collected at −153˚C (120 K) for 3b-3c and at −123˚C (150 K) for 3a.
The crystallographic data of these complexes were summarized in Table 1. APEX2 software was used for preliminary determination of the unit cell [29]. Determination of integrated intensities and unit cell refinement were performed using SAINT program [30]. The structures were solved with SHELXS-2014/7 [31] and subsequent structure refinements were performed with SHELXL-2014/7.
3. Results and Discussion
Complexes 3a, 3b, 3c and 3d were synthesized from the corresponding ligand 1,3-diphenyl-1,3-propanedione by complexation reaction with europium (III) chloride in the presence of pyridine, bipyridine, piperazine and bipiperidine as counter cations (Scheme 1). This reaction is a standard preparation procedure
![]()
Table 1. Crystallographic data for the complexes (3a, 3b, 3c and 3d).
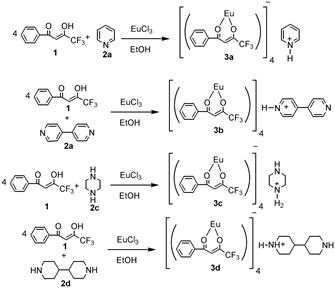
Scheme 1. Reaction scheme for the preparation of 3a, 3b, 3c and 3d.
for lanthanide (III) complex [32]. Structures of complexes were determined by mass spectrometry and X-ray single crystal structure analyses.
We have measured the UV-Vis and Fluorescence spectra of 3a, 3b, 3c and 3d. The UV-Vis and Fluorescence spectra of 3a, 3b, 3c and 3d were measured in solution phase (dichloromethane, 1 × 10−5 mol/L).
The fluorescence spectrum was measured in solution and solid state as well. The solution state fluorescence measurements were carried out in dichloromethane solution (1 × 10−3 mol/L). The corresponding absorption and emission spectrum of 3a, 3b, 3c and 3d were shown below (Figures 1-8, respectively). Complexes 3a, 3b, 3c and 3d exhibited absorption maxima at 327, 330, 326 and 313 nm, respectively. These strong absorption bands were assigned to the π-π* enol absorptions of the β-diketone ligand.
The fluorescence spectrum of 3a was measured by exiting the complex at 379 nm in solution and 307 nm in solid state. Strong emission band was observed from 600 to 630 nm. The complex 3b was exited at 379 nm in solution and 368 nm in solid state. Strong emission band was observed from 590 to 620 nm.
The fluorescence spectrum of 3c was measured by exiting the complex at 378 nm in solution and 307 nm in solid state. Strong emission band was observed from 600 to 650 nm. The complex 3d was exited at 377 nm in solution and 306 nm in solid state. Strong emission band was observed from 610 to 653 nm.
Suitable single crystals for X-ray structure analysis were easily obtained for all complexes. Since, europium (III) complexes are air stable, preparation of crystals is has been easy. All four complexes were dissolved in suitable solvents and left to slow evaporation at room temperature that resulted in crystals of complex 3a, 3b, 3c and 3d.
The complex 3a has 1,2-alternative structure, 3b has 1,3-alternative structure, 3c has cone like structure and 3d has partial cone like structure. In the crystal, complex 3a crystallized in monoclinic form with P21/n space group and it has four molecules in unit cell with pyridinium cation (Figure 9). The complex 3b also crystallize monoclinic form with P2/n space group and it has two molecules
![]()
Figure 2. Emission spectra of complex 3a. Blue and red color represents, spectrum in solution and solid phase, respectively.
![]()
Figure 4. Emission spectra of complex 3b. Blue and red color represents, spectrum in solution and solid state, respectively.
![]()
Figure 6. Emission spectra of complex 3c. Blue and red color represents, spectrum in solution and solid state, respectively.
![]()
Figure 8. Emission spectra of complex 3d. Blue and red color represents, spectrum in solution and solid state, respectively.
in unit cell with bipyridinium monocation. The complex 3c crystallized in monoclinic form with C2/C space group and it has sixteen molecules in unit cellwith piperazinium cation. The complex 3d also crystallized in monoclinic form with P21/n space group and it has two molecules in unit cellwith bipiperidinium cation.
The europium (III) ions of four complexes are coordinated by a distorted octahedral arrangement of eight oxygen atoms from four chelating β-diketone ligands (Figure 10). The average Eu1-O bond lengths are moderately normal, and these values are 2.384Å for 3a, 2.40 Å for 3b, 2.39Å for 3c and 2.38Å for 3d, respectively (Tables 2-5). The bond distances and bond angles are in good agreement with those reported for other analogous Eu-β-diketone complexes [33].
![]()
Table 2. Selected bond lengths (Å) and angles (˚) for the complex 3a.
![]()
Table 3. Selected bond lengths (Å) and angles (˚) for the complex 3b.
![]()
Table 4. Selected bond lengths (Å) and angles (˚) for the complex 3c.
![]()
Table 5. Selected bond lengths (Å) and angles (˚) for the complex 3d.
4. Conclusion
In conclusion, four new europium complexes have been synthesized and characterized. Further, molecular structures and photoelectronic properties of four europium complexes were determined. All four complexes exhibited strong emission between 590 - 640 nm, which could find prominent applications in light emitting devices. The absorbance and emission of the four complexes are quite the same. The fluorescence properties of all four crystals were very strong in solid state and very weak in solution state. These strong emissions were attributed to the 5D0 → 7F0-4 transition of Europium (III) ions under UV excitation. To further improve the scope of applications of these complexes, introduction of electron withdrawing groups such as -CN, -F on phenyl rings of fluorinated β-diketone ligand may improve the photoluminescence intensity and emission life time.
Acknowledgements
We are grateful to the Center for Instrumental Analysis, Kyushu Institute of Technology (KITCIA) for negative FAB-mass, and X-ray analysis.