1. Introduction
Organogels are a kind of nano-structured materials composed of a self-assembled superstructure of the low molecular weight gelators through non-covalent interactions including hydrogen bonds, hydrophobic interactions, π-π interactions, or Van der Waals forces and a large volume of organic liquid immobilized therein [1] [2]. Organogels are intensively investigated for different actual and emerging applications such as drug delivery [3], the templating of inorganic and polymer materials [4], dynamic gels [5], biological applications [6], etc. Recently, numerous efforts have been devoted to the development of stimuli responsive gels, whose properties can be either switched on-off or tuned in the presence of an external or internal chemical or physical stimulus such as light [7], thermal treating [8], ultrasound [9], and change in pH [10]. Such responsive gel systems are highly desirable for the development of sensor devices.
Anions play a crucial role in a wide range of chemical and biological processes. Among numerous anions, the fluoride anion is significantly important for health and environmental issue. For example, fluoride can be applied on dental care and treatment of osteoporosis, whereas superfluous intake of F− wound causes fluorosis [11]. Therefore, design and development of sensing and recognition for F− have grown into an area of great interest in supramolecular and biological chemistry in recent years [12] [13]. It is well-known that the NH units such as amides [14], urea [15], indole subunits [16] could be served as binding sites for anions in the dilute solution. Owing to the commonality of hydrogen bonding between organogels and anion-bonding supramolecular hosts, the organogel systems might show reversible color change and gel-sol transition by anion stimulus. Organogel showing both reversible color change and gel-sol transition by anion stimuli are limited although there are articles on anion-tuning organogels [12] [17] [18] [19].
Herein, we report the synthesis, self-assembly and fluoride-responsive behavior of the compound N-[4-(dodecyloxy)-3-(hydroxy)-benzoyl]-N’ (4’-nitrobenzoyl) hydrazide (D12, Scheme 1). D12 showed strong gelation ability in some organic solvents such as toluene, benzene and chloroform (Table 1). An FT-IR study confirmed that intermolecular hydrogen bonding was the major driving force for gelation of organic solvents. The D12 gel exhibited gel-sol transition and color change upon addition of F−.
2. Experiment Section
2.1. Synthesis of Compound
D12 was synthesized according to the route shown in Scheme 2. The synthesis of the compound was done according to the instruction of Schubert et al. [20]. The compounds of D12 were purified through repeated recrystallization from alcohol/water for further 1H NMR, FT-IR and elemental analysis.
1H NMR (400 MHz, DMSO-d6) (ppm from TMS): 10.76 (s, 1H), 10.38 (s, 1H), 9.21 (s, 1H) 8.37 (d, J = 8.71 Hz, 2H), 8.15 (d, J = 8.45 Hz, 2H), 7.37 (d, J = 7.74 Hz, 2H), 7.02 (d, J = 7.54 Hz, 1H), 4.02 (t, J = 6.27 Hz, 2H), 1.72 (m, J = 7.80, 7.18 Hz, 2H), 1.44 (m, J = 7.80, 7.24 Hz, 2H), 1.24 (m, 16H), 0.85 (t, J = 6.35, 6.84 Hz, 3H).
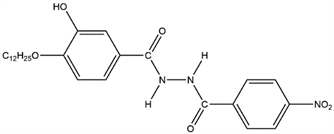
Scheme 1. Molecular of D12.
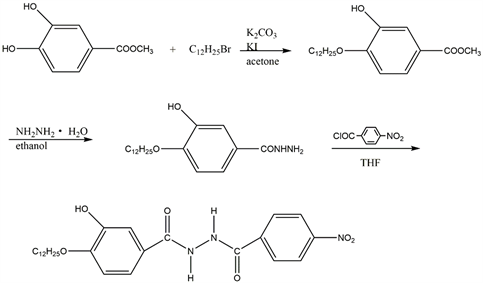
Scheme 2. The synthesis of compounds D12.
FT-IR (KBr disc cm−1): 3214 (ν-NH, -OH), 3025 (νAr-H), 2917 (νas-CH2-), 2850 (νs-CH2-), 1678 (νC=O), 1638 (νC=C), 1587 (νC=O), 1529 (νas-NO2), 1489, 1468 (δCH2), 1346 (νs-NO2), 1331, 1299 (νC-N, δN-H), 1168 (νO-C), 1047 (δAr-O), 1013, 870, 849 (δAr-H), 719 ((CH2)n, n ≥ 4).
Elem. Anal: Found: C 64.42%, H 7.31%, N 8.52%. Calcd for C26H35N3O6: C 64.31%, H 7.27%, N 8.65%.
2.2. Characterization
1H NMR spectra were recorded with a Bruker Avance 400 MHz spectrometer using tetramethylsilane (TMS) as an internal standard. Scanning electron microscopy (SEM) images were taken with SSX-550 apparatus. FT-IR spectra were recorded with a Perkin-Elmer spectrometer (Spectrum One B), the sample was pressed tablet with KBr, or cast film on the KBr discs. X-ray diffraction was carried out with a Rigaku D/max 2500 PC X-ray diffractometer (Cu Kα, λ = 1.5406 Å). Elemental analyses were carried out on a vario EL cube elemental. UV-vis absorption spectra were recorded on a Shimadzu UV-2550 spectrometry.
2.3. Gelation Experiments
A weighed powder sample was mixed with an organic solvent in a sealed test tube and the mixture was heated until the solid dissolved. The resulting solution was cooled to 4˚C and then the gelation was checked visually. When the tube can be inverted without fluid, we judged it “gelation”. Melting temperature (Tgel) was determined by the “falling drop” method [21]. The xerogels were obtained through slowly spontaneously evaporating the solvents from organogels, which were kept under the atmosphere at about 4˚C.
3. Results and Discussion
3.1. Gelation Properties of D12
The gelation properties of D12 have been tested in several different solvents and the minimum gel concentrations were summarized Table 1. As shown in Table 1, the gelator D12 could form stable gels in some tested solvents such as toluene, benzene, chloroform, but was insoluble in cyclohexane and was dissolved in dimethyl sulfoxide (DMSO).
![]()
Table 1. Gelation properties of D12*.
*G: gel; S: Solution; I: insoluble; P: precipitate; the minimum gelation concentration (wt%) was given.
In order to investigate the thermal behavior of the gels, we studied the relationship between the gel-sol transition temperature (Tgel) and the concentration of D12. Figure 1 showed the plot of Tgel versus the gelator concentration in toluene, benzene and chloroform.
To obtain visual images of the organogels from different solvents, the morphologies of the corresponding xerogels were investigated by scanning electron microscopy (SEM). As shown in Figure 2, the xerogels from toluene and chloroform consisted of fibers with an average diameter of 80 - 120 nm.
![]()
Figure 1. Melting temperature (Tm) of the gels based on D12 in different solvents.
![]()
![]()
Figure 2. SEM images of xerogels from (a) toluene (1.23 wt%) and (b) chloroform (1.19 wt%).
The self-assembly behavior was studied by FT-IR spectroscopy and X-ray diffraction (XRD). Figure 3 shows the FT-IR spectra of the xerogels from toluene and chloroform. Bands of N-H stretching vibrations of the acyl hydrazine group appear at 3178 cm−1 (toluene gel) and 3176 cm−1 (chloroform gel) as well as bands of C=O stretching vibration at 1672 cm−1 (toluene gel) and 1672 cm−1 (chloroform gel), indicating that the N-H of the acyl hydrazine group is associated with the C=O group via N-H…C=O hydrogen bonding in the xerogel [22], such hydrogen bonding interaction was considered to be the main driving forces in the gelation process. In addition, the absorption bands of the antisymmetric (υas) and symmetric (υs) CH2 stretching vibrational modes of D12 were observed at 2920 cm−1 (υas CH) and 2851 cm−1 (υas CH) in the gel respectively, indicating that the alkyl chains are ordered in part and there is a strong organization of the alky groups via van der Waals interaction [22].
Powder XRD measurements were performed on the xerogels to obtain further information on molecule arrangement in their gel phase. Figure 4 shows the
![]()
Figure 3. FT-IR spectra of xerogels from (a) toluene and (b) chloroform.
![]()
Figure 4. X-ray diffraction patterns xerogels of D12 in toluene.
X-ray diffraction patterns of D12 xerogel from toluene. The XRD pattern of D12 xerogel from toluene exhibited one strong peak and three weak peaks in the low-angle range. Their d-spacings are 47.22 Å, 15.83 Å, 11.46 Å and 9.48 Å, illustrating that a lamellar structure with 47.22 Å of interlayer distance in the gel phase. The interlayer distance is measured to be about 1.5 times of its molecular length (l = 30.21 Å). In the high-angle regions, three diffuse broad peaks with a maximum at about 3.7, 4.0 and 4.5 Å, suggesting the coexistence ordered and disordered feature. The peak at 4.5 Å is characteristic of the aliphatic chains, while that at 4.0 Å might be attributed to the repeat distance of hydrazide units in stacking [23]. A similar arrangement can also be observed for D12 xerogel from chloroform, indicating a similar supramolecular arrangement in both gel states. We proposed that molecules should self-assemble into a lamellar structure.
3.2. Fluoride Responsive Properties
The effect of fluoride anion on the organogel was revealed by the gelation experiment of D12 in toluene in the presence of F− (as [Bu4N]+ salt). On careful addition of solid [Bu4N]F・3H2O onto the top of the toluene gel, a thin layer of red solution immediately appeared at the upper part and then the gel gradually vanished and more and more red-colored solution appeared until all the gel transferred into solution. The whole process took place in no more than one minute (Figure 5). The presence of fluoride not only changed the color of the system but also disrupted the gel to a solution. The results indicated that D12 gel is sensitive to fluoride, with naked eye sensing by gel-sol transition and obvious color changes.
The interaction between D12 and the fluoride was investigated in detail through absorbance spectra titration experiments. Figure 6 shows the complete family of spectra during the titration of a 1 × 10−4 mol・L−1 solution of D12 in DMSO with TBAF. A new absorption band at 420 nm appeared upon addition of 1equid. F−, whose intensity reached the maximum value at the 10 equiv. of F−. The clear isosbestic point at 320 nm indicates that a single component is produced in response to the interaction between D12 and F− [24].
The interaction between D12 and fluoride was further investigated by 1H NMR titration experiments in DMSO-d6. As shown in Figure 7, the amide NH proton signal at 10.7 and 10.3 ppm disappeared and a weak broad signal at 10.2
![]()
Figure 5. The transparent organogel formed from a solution of D12 in toluene (2.56 wt%).
![]()
Figure 6. Absorbance spectra changes of D12 (1 × 10−4 mol∙L−1) upon the addition of 0 - 5 equiv. F−1 in DMSO.
![]()
Figure 7. Partial 1HNMR spectra of D12 (1 × 10−2 mol∙L−1) in DMSO-d6 upon the addition of different amount of F−.
ppm simultaneously appeared with increasing fluoride concentration from 1to 2 equiv. The appearance of the weak broad band at 10.2 ppm indicated that the N-H∙∙∙F− hydrogen bond formed. Upon addition of 3 equiv. of F−, a new weak broad signal appeared at16.0 ppm, which was ascribed to the [HF2]− dimer and suggested that a hydrazide N-H group might undergo a deprotonation process [25].
Based on the analyses of UV-vis absorption and 1H NMR spectra, it can be concluded that there are two stepwise equilibria in this responsive process. Upon the addition of the lower equiv. F−, the proton was abstracted slightly from the hydrazide subunit and resulted in the formation of 1:1 supramolecular complex [D12・F]−, as shown in the following equilibrium (1). On the addition of the higher equiv. F−, the base becomes very strong, and F− exhibits a large affinity toward H+, which induces the deprotonation of the [D12・F]− complex to form the very stable [HF2]− dimer, as illustrated by equilibrium (2). Meanwhile the charge-transfer might result in a five-membered ring based on intramolecular hydrogen bonding between the oxygen atom near the deprotonation nitrogen atom and the other NH could thus be formed. Thus, the extended conjugated system was formed through the adjacent phenyl group and a five-member ring, which is responsible for the dramatic color change upon the addition of fluoride ions [25].
(1)
(2)
4. Conclusion
A novel gelator D12 was synthesized, and showed strong gelation abilities in some of the tested solvents. It is demonstrated that molecules self-assembled to form gels consisting of fibers mainly through intermolecular hydrogen bonding and the van der Waals interactions. The layered arrangement was found in the fibers. The gel systems exhibited gel-sol transition and color change in response to F−. A possible mechanism for the F− responsive process was proposed. Upon the addition F−, one of the N-H group undergoes a deprotonation, the other N-H group together with the oxygen atom nearby deprotonation nitrogen atom to form intramolecular hydrogen bonding. It destroyed the intermolecular hydrogen bonding and subsequently led to the change of gel-sol. Meanwhile, the color change of the gel was mainly attributed to the extended conjugated system formed through the adjacent phenyl group and a five-membered ring.
Acknowledgements
The authors are grateful to Project of Science and Technology Plan in Shenzhen City (Project No. JCYJ20180305125649693).