1. Introduction
Mango fruits are commercialized worldwide for their sensorial and nutritive qualities, antioxidant and dietary properties [1] [2]. Top producers are Asian countries such as India, China, Thailand, Indonesia and Pakistan. These countries produced 15,188,000 to 1,888,449 MT of mango in 2014 (FAOSTAT Database), compared to Reunion Island that produces around 3500T of mango a year, both for exportation and local market use [3]. At preharvest stage, mango fruits suffer attack from fruit fly (Bactrocera dorsalis, Tephritidae), bacterial (Xanthomonas, Xanthomonadae; Ralstonia, Rasltoniaceae) and fungal pathogens (Penicillium, Trichocomaceae; Alternaria, Pleosporaceae; Fusarium, Nectriaceae and Colletotrichum, Glomerellaceae) that induce visual damages like rots and lesions at postharvest stage and cause tremendous loss during storage [4] [5]. One of the biggest challenges for the mango market is to protect stored fruit against anthracnose development. Postharvest anthracnose is a disease caused by phytopathogenic fungi included in the Colletotrichum genera that reduces mango fruit marketability, storability and nutritional value [6]. Current treatments combine one to multiple methods to overcome postharvest loss caused by phytopathogenic fungi: copper sprays can be applied at the preharvest stage, hot water treatment that is for use before storage; carbendazim treatment is used during storage, whereas prochloraz and benomyl are used as postharvest treatments [7]. Most of these treatments are of a chemical nature. There is thus a need for an eco-friendly and biological alternative product that can be used during mango storage to prevent anthracnose incidence and postharvest loss.
Essential oils (Eos) are natural products known through ancient time to have protective abilities against food spoilage and therefore were incorporated in stored food for their antibacterial [8] and antifungal [9] activities against a broad range of animal, human and plant pathogens, thus extending their shelf life of fruits without any negative effects on their physiochemical and sensorial qualities [10]. Eos such as cinnamon Eo [11], thyme Eo [12] [13] [14] and clove oil [15] are among the most investigated and successful against fruit postharvest pathogens. Their effects against Colletotrichum genera are generally focused on their in vitro ability to inhibit fungal growth and spore germination. Clearly, their effectiveness against the anthracnose disease pathogen and its usability as a mango preservative would benefit from further investigation.
Many papers link the ability of Eos to induce fruit resistance against Colletotrichum infection to their capacity to elicit an effect on defense-related compounds such as resorcinol, chitinase enzyme synthesis in tropical fruits [16] [17]. In particular, researches on mangoes linked fruit maturity, resistance to phytopathogenic fungi to a decrease in resorcinol synthesis in mango peel [17] [18]. Such findings support the hypothesis that resorcinol compounds are involved in mango resistance to phytopathogenic fungi-caused disease.
Malagasy essential oils have recently been reported to have antifungal properties, present paper focused on two of them: clove and ravensara Eos. Clove trees were introduced into Madagascar just before the colonial period. These Eo-producing trees are actually well domesticated and play a predominant role in the economy of eastern Madagascar [19], whereas Ravensara aromatica Sonnerat (also known as Cryptocarya agathophylla Van der Werff) is an endemic tree located in the central and eastern parts of the island [20]. Clove Eo ability to inhibit phytopathogenic fungal germination and growth is already established worldwide [21]. In addition to its antifungal properties, clove Eo was also found to have antioxidant [22] and protective abilities against oxidative/nitrosative stress [23]. Similarly, clove Eo and Ravensara Eo from Madagascar are both reported to have a growth inhibition effect on human and plant pathogens such as Aspergillus niger and Saccharomyces cerevisiae [24], in addition to their phytotoxic [25] and antioxydant properties [26], and studies on the chemical composition and physical properties of both Eos [20] [27] [28] reported comparable results with previous investigations on the subject [29] [30].
This study is therefore based on the hypothesis that native Malagasy Eo also has antifungal abilities that can be used to prevent anthracnose development on stored mangoes from Reunion Island. The high variability and volatility of Ravensara Eo [31], on which its anti-germinative intensity depends [25], and the microvariability and high density of clove Eo [32] are important characteristics on which investigations were focused. Experiments were then conducted on the biological activities of these Eos against Colletotrichum and their effect on mango fruit anthracnose development and resorcinol production in view of their eventual use as a potential mango preservative during postharvest storage.
2. Materials and methods
2.1. The Collection of Essential Eos and Their Analysis
Five Eos extracted by hydrodistillation from fresh leaves in Madagascar were used in this study: one clove (Syzygium aromaticum L.) Eo and four chemotypes of Ravensara aromatica Sonnerat (also named Cryptocarya agatophylla Van der Werf) Eos: methyl chavicol (Type MC), methyl eugenol (Type ME), limonene (Type L) and sabinene chemotypes (Type S).
Eos compositions were determined by GC-MS analysis. The GC/MS analysis was conducted on a CLARUS 480+ gas chromatograph and an Elite-5MS column (length: 60 mm; I.D.: 0.25 mm). Compounds were identified by comparing the collected mass spectra with NIST08 (National Institute of Standards and Technology) database and their proportion in the Eo was established from each component’s pic area in the chromatogram.
2.2. Fungal Pathogens and in Vitro Toxicity Assessment
Mango fruit anthracnose-isolated Colletotrichum asianum strains (MUC43868) were obtained from a Belgian collection (Université catholique de Louvain) and used for in vitro studies and to induce anthracnosis on mango fruits produced in Reunion Island.
2.2.1. Toxicity against Conidial Germination
Thirty conidia from a 15-day old culture of C. asianum were deposited on solidified Potato Dextrose Agar (PDA) Petri plates. The plates were reversed and 0, 10, and 20 µL of Eos were added to the plate lid (making 0, 112.5 and 225 µL/L of air concentration) prior to sealing each of them with a parafilm so as to to prevent any Eo flux between plates or any contact between the Eo and the conidia during incubation. All plates were incubated at 27˚C (optimal temperature for C. asianum growth in vitro). Ten replicates were used for each treatment. Germinated conidia were counted daily (8 a.m.) for 7 days and final germination was recorded and expressed as:
In order to determine if germination was prevented or stopped at its early stages, optical studies were conducted on all treatments that induced no visible germination by daily inspection of the Petri plates under an optical microscope.
2.2.2. Toxicity against Mycelial Growth
1 mm2 mycelial plug from a 15-day-old culture of C. asianum was grown on PDA plates with 0, 10, and 20 µL of Eos as in (2.2.1).
Radial growth was observed everyday (8 a.m.) for 7 days and the final mycelial diameter (MD) was recorded. Plugs with no growth were recultivated on freshly and the final mycelial diameter (MD) was recorded and expressed as:
Criteria adopted by Bill et al. [33] were used to discriminate the effect of tested EO on C. asianum: treatments that induced no mycelial growth were defined as fungicidal, while treatments that showed mycelial growth inhibition was fungistatic.
2.3. Bioactivity of Essential Eos on Mango Fruits Assessment
2.3.1. Effect of Eos on Anthracnose Development
12 Mango fruits (Cogshall var.) were first weighed, cleaned under running water and sterilized with 90˚ ethanol. Half of them were then inoculated with 10 µL of C. asianum spore suspension (105 conidia per mL) and maintained at 20˚C for 48 hours: a drop of conidia suspension was deposited on each mango, a small paper circle was placed over it, and each piece of paper was covered with a water-soaked cotton pad (two inoculating sites were determined for each fruit). Prior to incubation with Eos, the piece of paper and the cotton were removed.
Two closed plastic boxes (15 L contenance) were lined with aluminum foil (to prevent the Eo from permeating the boxes). In one of them, 500 µL of clove Eowere sprayed on the inner surface using 50 µL droplets of Eo (making 30 µL/L of air concentration). Ten of the above mangoes were transferred to each box and were incubated at 20˚C (storage temperature adopted by local producers and wholesalers). After 1 day of incubation, the aluminum foil coating was removed from the container with the Eo. Each box was kept open, as was the incubation chamber until all of the Eo scents had evaporated. The incubation chamber was then closed.
Lesion area (LA) is expressed as the mean of lesions observed in the two inoculated zones which are measured by their length (L1 and L2) and their width (l1 and l2) at the ripened stage of all mangoes:
2.3.2. Effect of Eos on Active Defense Response-Related Compound Content in Mango Fruit
Twenty-five mangoes were cleaned as specified above (2.3.1). For sampling, mango peels were removed, wrapped in aluminum foil, immersed in liquid nitrogen, mixed to a powder using a Retsch ® Grindomix, and stored at −80˚C. The same preparation was done to square-cut mango pulp. Five out of 25 mangoes were sampled before incubation, while the remaining 20 were incubated with or without 500 µL of sprayed clove oil as in (2.3.1). Five treated mangoes and five untreated mangoes were sampled after EO-impregnated aluminium foil removal. Final samplings were done at the ripened stage (15 days after incubation) for the remaining five treated and five untreated mangoes.
Resorcinol content was measured from 0.5 g of mango peel powders lyophilized beforehand, as described in (Knödler et al. 2009), using an HPLC apparatus (Dionex® Ultimate 3000 apparatus-length: 250 mm; I.D.: 4.6 mm; 5 µm; 30˚C stationary phase; Symmetry Shield RP18 column equipped with a diode array involving two eluents [A: H2O: CH3CN (99.8: 0.2, 0.01% HCOOH) and B (CH3CN 100%)]). The gradient program was also adapted from (Knödler et al. 2009) (see Table 1). The detection of the AR was at 275 nm. Each compound was quantified and identified by comparison with a commercial standard of resorcinol (Sigma Aldrich). Pulp color (L, a*, b* indices) was measured using a Minolta ® C-400 chromameter in order to calculate °hue saturation. Freshly frozen ground pulp was used to measure total titratable acidity (ATT in meqv/100g MF), pH and total soluble solid content (measured in °Bx).
![]()
Table 1. HPLC gradient program for AR quantification (%).
2.4. Identification of Active Compounds in Clove Eo
In order to find out if clove Eo fungitoxicity was due to its major component (eugenol) or to the synergism between its components, eugenol was purchased from a local producer (CTHT: Centre de Technique Horticole de Tamatave) and submitted to fungitoxicity tests on conidial germination and mycelial growth, anthracnose lesion area. Doses were adjusted to the eugenol content in the clove Eo tested (8.1 and 16.2 µL for in vitro assays, 405 µL for in vivo assays i.e. 91.125, 185.25 and 24.3 µL/L of air concentration).
2.5. Statistical Analysis
Variance analysis (ANOVA), using XLSTAT software, was used to compare the effects of each treatment on conidial germination, mycelial growth, lesion area, fruit quality and resorcinol content. Tuckey post hoc test was used to enlighten significant differences amongst the effect of each treatment on measured parameters. In Figure 1 and Figure 2 and Tables 3-5, values with the same letter belong to the same homogeneous group.
3. Results
3.1. Essential Eo Composition
GC-MS analysis provided the EO compositions represented in Table 2. Clove Eo consisted essentially of eugenol (81%) and caryophyllene. The four Ravensara Eos contained similar minor components and differed in the major components and their proportions: 85% of methyl chavicol (estragole) for the first Eo, 53% of D-limonene for the second Eo, 70% of methyl eugenol for the third Eo and 28% of sabinene for the last Eo. Therefore, the collected Ravensara Eos belong to 4 chemotypes: an MC one, an ME one, an L one and an S one.
3.2. In Vitro Effects of Essential Eos on Colletotrichum Asianum
Similar to most reports on Eos, including clove Eo effects on Colletotrichum species, our results confirmed that clove Eos from Madagascar’s eastern forests
![]()
Figure 1. Effect of clove oil and R. aromatica oils (112.5 and 225 µL/L of air) on the percentage of conidial germination. GM stands for Malagasy clove oil, MC for methyl chavicol chemotype of R. aromatica oil, ME for methyl eugenol chemotype of R. aromatica oil, L for limonene chemotype of R. aromatica oil and S for sabinene chemotype of R. aromatica oil. Means followed by a common letter above each column are not significantly different at the 5% level.
![]()
Figure 2. Effect of clove oil and R. aromatica oils (112.5 and 225 µL/L of air) on the percentage of mycelial growth. GM stands for Malagasy clove oil, MC for methyl chavicol chemotype of R. aromatica oil, ME for methyl eugenol chemotype of R. aromatica oil, L for limonene chemotype of R. aromatica oil and S for sabinene chemotype of R. aromatica oil. Means followed by a common letter above each column are not significantly different at the 5% level.
havea strong fungitoxic activity against C. asianum while effects of R. aromatica Eos were fungistatic. All tested clove Eo achieved complete inhibition of conidial germination of C. asianum. On the other hand, significant decreases of (P < 0.05) were only observed with 225 µL/L of methyl eugenol chemotypes and all tested sabinene chemotype of R. aromatica Eo, while limonene and methyl chavicol chemotypes of R. aromatica Eos showed no effect at all (see Figure 1and Photo 1).
Every tested Eo showed a significant (P < 0.05) decreasing effect on the mycelia
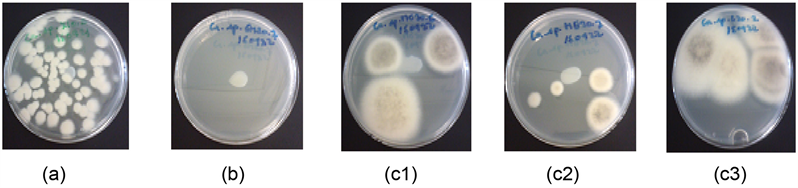
Photo 1. Conidial germination of C. asianum (a): without Eo; (b) With 20 μL of clove Eo; (c1) With 20 μL of Methyl chavicol chemotypes of R. aromatica Eo; (c2) With 20 μL of Methyl eugenol chemotypes of R. aromatica Eo; (c3) With 20 μL of Limonene chemotypes of R. aromatica Eo.
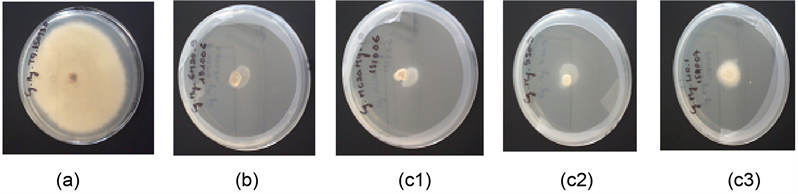
Photo 2. Mycelial growth of C. asianum (a): without Eo; (b) With 20 μL of clove Eo; (c1) With 20 μL of Methyl chavicol chemotypes of R. aromatica Eo, (c2) With 20 μL of Sabinene chemotypes of R. aromatica Eo, (c3) With 20 μL of Limonene chemotypes of R. aromatica Eo.
growth of C. asianum. The methyl chavicol chemotype of R. aromatica Eo and clove Eo totally inhibited mycelial growth of C. asianum. Regardless of its ineffectiveness against C. asianum conidial germination, the methyl chavicol chemotype of R. aromatica totally inhibited C. asianum mycelial growth (see Figure 2 and Photo 2).
Therefore, the methyl chavicol chemotype of R. aromatica Eo also had significant fungicidal activity against mycelial growth, although its ineffectiveness against conidial germination prevents it from being the best choice for a mango anthracnose preservative. In the same range, clove Eo showed a greater inhibiting effect than the methyl eugenol chemotypes of R. aromatica Eo, whereas the eugenol content of tested clove Eo and the methyl eugenol content of Ravensara aromatica Eo are similar.
3.3. Essential Eo Treatment and Its Effect on Mangofruit Metabolism and Defense-Related Compounds
Clove Eo treatment (30 µL/L) induced a significant effect on the development of anthracnose in artificially inoculated mangoes (Table 3). Lesion area significantly decreased from untreated to treated mangoes at a P value < 0.05. The treatment did not alter the physical quality of mangoes pulp. No significant differences were observed on pH, total titratable acid content, total soluble solid content, weight loss and pulp color from treated to untreated mangoes at a P
![]()
Table 2. Chemical composition of the Eos tested (%).
value < 0.05.
5-pentadecylydroresorcinol content also showed no significant difference between treated and untreated mango peel samples (P < 0.05, see Table 4).
3.4. Identification of Active Compounds
Colletotrichum asianum was found to be similarly inhibited with eugenol as with clove Eo. One hundred percent inhibition was recorded on its mycelial growth and conidial germination, with all tested doses of eugenol (91.125 and 185.25 µL/L of air). Such results suggest that clove Eo fungitoxicity is not due to synergistic activities between its components but to an active compound, the
![]()
Table 3. Effect of malagasy clove Eo on anthracnose development, and physical characteristics of mango fruit 15 days after Eo treatment.
![]()
Table 4. Effect of Malagasy clove Eo on resorcinol (5-pentadecylydroresorcinol) content (mg/g of fresh peel).
major component of clove Eo: eugenol. The same effects were observed on anthracnose development when 405 µL of eugenol (i.e. 24.3 µL/L of air) induced a significant inhibition of lesion areas in ripening mango fruits (see Table 5). The recorded inhibition was slightly less than with clove Eo, though statistical analysis indicated that the effects of clove Eo and eugenol on lesion areas of ripening mangoes belong to similar groups (a and ab).
4. Discussion and Conclusion
The essential Eo compositions established on the basis of GC-MS are in accordance with previous reports on Malagasy clove Eoand Ravensara aromatica Eo compositions [27] [28], as well as with reports on other clove Eo compositions [29] [30]. The clove oil was mostly constituted of Eugenol and Caryophyllene and Ravensara oils exhibited similar components but varying amounts of each component. Each R. aromatica oils collected had one major component. Therefore, the collected Eos belong to 4 chemotypes.
Similar to its phytotoxic effects [25] and to the antibacterial effect of Pimenta racemosa var. racemosa leaf Eo against tomato wilt [34], the antifungal properties of Ravensara aromatica Eo also vary with the Eo chemotypes. Therefore, the antifungal activity of different doses of major components should be compared in order to confirm such a hypothesis. Since most antifungal activity reported is dose-dependent [35] [36] [37], and minor components of Eos are also known to have strong toxic abilities [9], this Eo chemotype-dependence of Ravensara aromatic Eo cannot solely be attributed to major Eo components, especially in view of Prakash’s findings on the negative effects of minor compounds on eugenol
![]()
Table 5. Effect of eugenol on mycelial growth and conidial germination of C. asianum and on anthracnose development in mango fruit.
toxicity [38]. Difference between clove oil in vitro toxicity and ME-typed ravensare Eo corroborated the previous findings on the decreasing effect of methylation of eugenol on its biological activities [39].
Many authors found Eo treatment to be significantly effective against phytopathogenic fungi-caused diseases [40] without altering the physico-chemical properties of mangoes [41]. Moderate preventive effects of sprayed clove oil were reported by Santamarina et al. on stored rice grain [42] while complete control of Aspergillus flavus, Penicillium cinitricum caused disease on oranges and jujube fruits were reported by Xing et al. [43]. Bill et al. [33] also reported strong curative effects of thymol oil fumigated on artificially inoculated avocadoes and demonstrated that such an effect can be partially attributed to the oil’s ability to elicit resistance compounds release such as chitinase, glucanase and total phenolic compounds without altering fruit marketability. Our investigation revealed similar inhibitory effects concerning the ability of clove oil to prevent anthracnose on mango fruit in storage conditions, but no ability to induce the synthesis of resorcinol compounds was found in mango peel although our results corroborate previews statement on resorcinol content’s decrease with ripening process [44]. Such findings are in agreement with the in vitro effects of clove oil treatment where mycelial growth and conidial germination were totally inhibited with 20 µL of clove oil. The present results suggest that inhibition of anthracnose development in ripening mangoes was mainly due to the toxic effect of clove Eo on C. asianum growth since more research is needed to prove that clove Eo has no effect on the internal resistance of mango to anthracnose. Some researchers directly applied the Eo on the fruit using the pulverization method or by incorporating the Eo into an edible coating such as aloes vera gel and chitosan [45]. Bautista-Banos et al. [46] and Bill et al. [33] reported that these techniques led to a greater reduction in lesion area than current commercial fungicides on fruit anthracnose.
Some works found in the literature also report that essential oil treatments have strong antifungal activity in vitro but weak in vivo and thus do not induce significant inhibition on disease severity or defense-related enzyme activity. Shao et al. [47] reported similar findings when using clove oil on citrus green mold. Itako et al. also found that cymbopogon oil strongly inhibited spore germination in vitro, whereas sporulation and appressorium formation was not significantly reduced on sprayed leaves [48].
The present work attributes the toxicity of clove oil against mango anthracnose and its pathogen development to its major active compound, eugenol. Most research on the identification of the active compounds of a product is in agreement with such findings [42] [49] [50] [51] even if rare synergistic effects between components of the Eo have been reported. On the contrary, Prakash et al. [38] found antagonistic activities between eugenol and the remaining compounds of Piper betle L. essential oil to combat moisture in some edible commodities. They reported that eugenol showed better antifungal activity alone than when it was incorporated into Piper betle L. essential oil.
Acknowledgements
The authors are grateful to UMR QUALISUD of CIRAD REUNION, DP Forêts et Biodiversité (a joint program between the University of Antananarivo, CIRAD and FOFIFA), the French Embassy, the PARRUR Project and the AFS4FOOD Project for providing the necessary funds and facilities to conduct this research.