Synthesis and Crystal Structure of Bis(N’-(Pyridine-3-Carboxaldehyde) Isonicotinoylhydrazone) Zinc(II) ()
1. Introduction
There is a substantial increase in the coordination chemistry of zinc due to its structural diversity. Zinc(II) can form complexes with a flexible coordination environment with geometries ranging from tetrahedral, trigonal bipyramidal and square pyramidal to octahedral [1] [2] [3] [4]. Zinc(II) complexes have been found to play a variety of roles in living organisms, such as catalytic cofactor for a number of metalloenzymes, stabilizing protein structures and modulating the interactions between macromolecules [5] [6] [7].
Schiff bases are important as ligands because of their ease of preparation and their resemblance to natural biomolecules [8] [9]. Their metal complexes have been studied extensively because of their attractive physico-chemical properties and structural diversity, which account for their wide range of applications in industry and biology [9] - [13]. Zinc complexes of Schiff base ligands have received particular attention due to their ease of preparation, high thermal stability, photoluminescence properties and their potential applications as catalysts and model molecules for biochemical systems [10] [12] [14].
While Heterocyclic nitrogen containing compounds such as pyridine and its derivatives are present in many synthetic and natural systems, heterocyclic Schiff base ligands containing O-, N- or S-donors and their metal complexes exhibit interesting properties [7] [15] [16]. The study of the structural chemistry of Schiff base ligands and their complexes is important in understanding the complex biological properties of these systems. Such ligands have been used to construct various metal-organic frameworks including coordination polymers due to their capabilities to act as chelators or connectors using the imine linkage [9] [17].
Our group recently embarked on studies on the biological activities of complexes of heterocyclic Schiff base ligands [13] [18]. The observed biological activities result from the presence of the characteristic azomethine (-N=CH-) functionality of the Schiff base. One such class of heterocyclic Schiff base ligands are pyridinecarboxaldehyde isonicotinoyl hydrazones, derived from isoniazid (Isonicotinic acid hydrazide), which generally are bidentate or tridentate chelators and typically form six-coordinate complexes [19] [20] [21] [22]. We report here the synthesis, characterisation and X-ray single crystal structure of a zinc(II) complex of N’-(pyridine-3-carboxaldehyde)isonicotinoylhydrazone Schiff base ligand in which bonding to the zinc atom is through the N-atom of pyridine while the imine group remains unusually uncoordinated.
2. Experimental
2.1. Materials and Measurements
All reagents were of analytical grade and were used without further purification. Elemental analyses (C, H, N) were determined on a Thermo Scientific FLASH 2000 Organic Elemental CHNS-O Analyser. The metal content was determined using a Thermo Scientific iCAP 6000 SERIES duo ICAP spectrometer. 1H-NMR and 13C-NMR spectra were recorded on a Bruker 400c instrument. Thermogravimetric analysis was carried out on a Mettler Toledo TGA/DSC1 Star System. Infrared spectra were recorded on a Thermo Scientific Nicolet iS5 (iDR ATR) spectrometer. The UV/Vis spectrum was recorded on an Agilent HP8453 Diode Array UV/Vis spectrometer. The single crystal X-ray structure was determined on a Bruker APEX diffractometer.
2.2. Synthesis of the Ligand, N’-(Pyridine-3-Carbaxaldehyde) Isonicotinoylhydrazone (L)
The ligand was prepared by a slight modification of a previously published method [19] [20] as illustrated in Scheme 1. Pyridine-3-carboxaldehyde (10,711 g, 100 mmol), in methanol was added dropwise to a stirred solution of Isonicotinic acidhydrazide (isoniazid) (100 mmol, 13,711 g) in hot methanol and the mixture was heated under reflux for 3 h. The resulting yellow precipitate was washed with hot methanol and dried in air at room temperature. Yield: 92%. Mpt: 232˚C - 234˚C Anal. Calc. for C12H10N4O: C 63.71; H 4.46; N 24.77. Found C: 63.77, H 4.27, N, 24.80; IR (KBr/cm−1): 3182w, 3005 - 2833w, 1681vs, 1557vs; 1H NMR (400 MHz, DMSO-d6): δ ppm 7.50 (dd, J7.89, 4.80 Hz, 1 H) 7.82 (d, J6.00 Hz, 2 H) 8.16 (dt, J7.99, 1.82 Hz, 1 H) 8.51 (s, 1 H) 8.63 (dd, J4.77, 1.61 Hz, 1 H) 8.79 (d, J6.00 Hz, 2 H) 8.88 (s, 1 H) 12.20 (br. s.,1 H).
2.3. Synthesis of Bis(N’-(N’-(Pyridine-3-Carbaxaldehyde) Isonicotinoylhydrazone) Zinc(II) [ZnL2(H2O)4](NO3)2·2H2O
A well stirred solution of the ligand, L (0.904 g, 4 mmol) in aqueous mixture was added drop wise to a stirred solution of Zn(NO3)2·6H2O (0.595 g, 2 mmol), dissolved in 2 mL of distilled water. The colourless solution of the metal salt turned yellow. The mixture was heated under reflux for 2 h and thereafter allowed to stand until the formation of light yellow crystals after two days. The crystals were filtered and dried in air. Yield: 40%. Mpt: 127˚C - 129˚C. Anal. Calc. for C24H32N10O14Zn: C 38.44; H 4.30; N 18.68; Zn 8.72. Found C 38.22; H 4.14; N 18.48; Zn 8.20. IR (KBr, cm−1): 3237br, 3237br, 3065 - 2973w, 1648s, 1557s.
1H NMR (400 MHz, DMSO-d6): 12.30 (2H, S, H(N3)), 8.89 (2H, S, H(C1), 8.80 (4H, d, J5.8 H(C(9), C(12), C(9’), C(12’)), 8.64 (2H, d, J3.8), 8.51 (2H, S, C(6)), 8.18 (2H, d, J8.0) 7.84 (4H, d, J6. C(10), C(11)), 7.52 (2H, dd, J8.0, 4.9, H(C(5), C(3)).
2.4. Crystal Structure Determination
A suitable pale yellow crystal of the title compound measuring about 0.14 × 0.11 × 0.05 mm3 was mounted using a glass fibre on the goniometer head of a Bruker APEX diffractometer and data were collected using graphite monochromated Cu-Kα radiation (λ = 1.54178 Å) at a temperature of 100 K. The structure was solved by Direct Methods and refined by full-matrix least squares on F2 [23]. All non-Hydrogen atoms were refined anisotropically. Hydrogen atoms were included in calculated positions, assigned isotropic displacement parameters and allowed to ride on their parent carbon atoms. All calculations were carried out using the SHELXTL package [24]. Table 1 presents the crystal data and refinement parameters; Table 2 lists the selected bond lengths and bond angles while Table 3 gives the hydrogen bond parameters. CCDC 1419801 contains the supplementary crystallographic data for this paper. This data can be obtained free of charge from the Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/dada_request/cif.
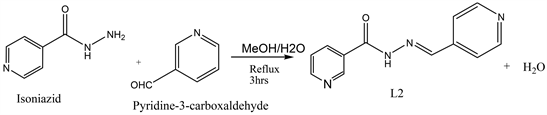
Scheme 1. Synthesis of N’-(pyridine-3-carbaxaldehyde) isonicotinoylhydrazone (L).
![]()
Table 1. Crystal data and structure refinement for [ZnL2(H2O)4](NO3)2·2H2O.
![]()
Table 2. Selected Bond lengths [Å] and Bond angles [˚] for [ZnL2(H2O)4](NO3)2·2H2O.
![]()
Table 3. Hydrogen bond Parameters for [ZnL2(H2O)4](NO3)2·2H2O.
3. Results and Discussion
The ligand, N’-(pyridine-3-carboxaldehyde) isonicotinoylhydrazone (L), was obtained from the condensation reaction between isoniazid and pyridine-3-carboxaldehyde in methanol to give a yellow precipitate. The precipitate is stable in air, soluble in DMSO and in hot aqueous alcohol. The reaction of N’-(pyridine-3-carboxaldehyde)isonicotinoylhydrazone and Zn(NO3)2·6H2O in a 2:1 molar ratio, in aqueous methanol mixture yielded a pale yellow powder and pale yellow rectangular-shaped crystals of [ZnL2(H2O)4](NO3)2·2H2O extracted from the filtrate. Both the precipitate and the crystals are stable in air. The crystals are soluble in DMSO and aqueous alcohol.
1H-NMR spectra were recorded in DMSO-d6 at 400 MHz to explore the variations of chemical shifts between the ligand and its complex. The 1H-NMR signals of the complex are shifted to higher frequencies as compared to that of the ligand (Figure 1). However, the resonance at 8.51 ppm, attributed to the proton at the N=CH imine functional group is the same in both the ligand and the zinc complex, thus indicating that the imine group does not participate in bonding.
![]() (a)
(a) ![]() (b)
(b)
Figure 1. (a) 1H NMR of L; (b) 1H NMR of [ZnL2(H2O)4](NO3)2·2H2O.
The 13C NMR spectrum of the free ligand displays a set of ten peaks due to the free rotation of the hydrazone arm (Figure 2(a)). The 13C NMR spectrum of the complex shows a set of ten peaks, suggesting a symmetrical structure (Figure 2(b)). Moreover, a comparison of the spectrum of the free ligand with that of the zinc complex shows a shift in the resonances towards higher chemical shifts upon coordination to the metal centre. This shift is even more intense for the carbon atom in the para position to the nitrogen atom of the pyridine ring. Such variations can be explained by the decrease of the shielding effect of the nitrogen atom. Therefore, all these observations fit and confirm the model where the zinc atom binds through the nitrogen atom of the pyridine, and where the imine group does not participate in bonding.
![]() (a)
(a)![]() (b)
(b)
Figure 2. (a) 13C NMR of L; (b) 13C NMR of [ZnL2(H2O)4](NO3)2·2H2O.
The IR spectrum of the ligand (L) and that of the ZnL2 complex show a broad peak at 3237 cm−1 attributed to υ(O-H) of water molecules in the complex which is clearly absent in the spectrum of the ligand (Figure 3). The peak at 3183 cm−1 corresponds to υ(N-H) stretching of the ligand. This peak is found to merge with the υ(O-H) band in the spectrum of the complex due to hydrogen bonding [25]. The υ(=C-H) band of the pyridyl ring is observed in the range of 3005 - 2833 cm−1. These bands are seen to shift to higher frequencies in the spectrum of the complex. The υ(C=O) band is seen to shift to a lower frequency in the spectrum of the complex due to extended conjugation with the nearby pyridine ring [10] [15]. The υ(C=N) band is observed at about the same position in both spectra indicating that this unit does not participate in bonding.
The UV/Vis absorption spectrum of the compounds was recorded at room temperature in DMSO as shown in Figure 4. The ligand exhibits a band at 301 nm, which is attributed to π-π* transitions of the pyridyl rings. On the other hand, the spectrum of the ZnL2 complex exhibits two bands. The first band at 277 nm corresponding to π-π* transitions of the pyridyl ring while the second band at 346 nm is attributed to a metal-to-ligand charge transfer transitions (MLCT). No absorption peaks were observed in the visible region as expected for a d10 system.
![]() (a)
(a)![]() (b)
(b)
Figure 3. (a) IR Spectrum of the Schiff base ligand (L); (b) IR Spectrum of [ZnL2(H2O)4](NO3)2·2H2O.
![]()
Figure 4. UV/Vis Spectrum of L and [ZnL2(H2O)4](NO3)2·2H2O.
3.1. Thermal Analysis
The thermal behaviour of [ZnL2(H2O)4](NO3)2·2H2O is shown in Figure 5 and represents the TGA thermogram recorded under nitrogen atmosphere in the temperature range of 30˚C to 600˚C at a heating rate of 25˚C·min−1 and consists of a three-step decomposition process. The first decomposition of 4.59% (calc. 4.8%) at 126.4˚C is attributed to the loss of the two molecules of the water of crystallization. The second step at 139.3˚C represents to the loss 10.4% (calc. 10.1%) corresponding to the decomposition of the two nitrate ions. The loss at 298.2˚C of 67.9% (calc. 68.6%) is attributed to the decomposition of the two ligand molecules and one water molecule. The residue of 17.5% which starts appearing at 350˚C probably corresponds to the decomposition of the remaining water molecule to leave a solid mixture which includes zinc oxide.
3.2. Scanning Electron Micrographs (SEM)
The surface morphology of materials is important for technical applications requiring well-defined surface or interfaces. The SEM images of the Schiff base ligand (Figure 6(a)) reveal a non-homogenous morphology of irregular particles of different sizes and shapes. The particle sizes range from 31.10 - 141.3 μm. The [Zn(L2)2(H2O)4](NO3)2·2H2O (ZnL2) complex displays loosely packed particles of different sizes and shape ranging from 503.5 nm - 2.04 μm (Figure 6(b)).
3.3. Crystal Structure of [ZnL2(H2O)4](NO3)2·2H2O
The ORTEP drawing of [ZnL2(H2O)4](NO3)2·2H2O showing its structure together with the atom numbering scheme is depicted in Figure 7. [Zn(L2)2(H2O)4] (NO3)2·2H2O has a symmetrical structure that crystallizes in the triclinic system with space group, P-1 as shown in Table 1. There is one crystallographically independent Zn(II) ion, two N’-(pyridine-3-carboxaldehyde) isonicotinoylhydrazone ligands, as well as four coordinated water molecules, in the symmetric unit. The important bond distances and angles are given in Table 2. The hydrogen bond parameters are given in Table 3. The molecular structure of the complex shows the zinc atom bonded to two molecules of N’-(pyridine-3-carboxaldehyde)isonicotinoylhydrazone Schiff base and four aqua ligands, with each Schiff base coordinating through its pyridine N-atom to form a distorted octahedral ZnN2O4 complex. The zinc(II) atom is therefore bonded to N1 of one ligand and the symmetry-related N1A of the other ligand and four oxygen atoms from the water molecules (O5, O5A, O6, O6A), giving a hexa-coordination around the Zn(II) center. The intermolecular hydrogen bond interactions in the complex are presented in Table 3. The coordination network is stabilized by intermolecular hydrogen bonding (O-H … O-N-O … H-O), resulting from two nitrates and two water molecules in the crystal lattice (Figure 8) [16]. This intermolecular hydrogen bond interaction leads to self-assembly of monomeric cores to form a supramolecular structure as presented in the packing diagram in Figure 9. The monomer of one complex therefore is linked to the monomer of another complex through intermolecular hydrogen bonding, involving coordinated water ligands and uncoordinated nitrates as shown in Figure 2. The two nitrate anions in the crystal structure neutralize the charge of Zn(II) ions.
![]()
Figure 5. TGA thermogram of [Zn(L2)2(H2O)4](NO3)2·2H2O (ZnL2) Complex.
![]() (a)
(a)![]() (b)
(b)
Figure 6. (a) SEM images of the ligand L; (b) SEM images of [Zn(L2)2(H2O)4](NO3)2·2H2O.
![]()
Figure 7. Ortep diagram of the molecular structure of [ZnL2(H2O)4](NO3)2·2H2O.
![]()
Figure 8. Diagram showing hydrogen bonding in [ZnL2(H2O)4](NO3)2·2H2O.
![]()
Figure 9. Packing diagram of [ZnL2(H2O)4](NO3)2·2H2O.
The bond lengths (Zn-N of 2.151 Å and Zn-O of 2.0738 - 2.125 Å) and the bond angles (N-Zn-N of 180˚ and O-Zn-O of 180˚ by symmetry) are close to values obtained for similar octahedral zinc(II) complexes [26]. The bonding of N’-(pyridine-3-carboxaldehyde) isonicotinoylhydrazone to zinc is in contrast to similar metal(II)-pyridine substituted isonicotinoylhydrazone schiff base complexes, which form typically 4- or 6-coordinate complexes in which each Schiff base ligand is coordinated through its pyridine N-atom, imine N-atom and carboxylate O-atom [20] [21] [22].
4. Conclusion
We have isolated a metal-organic framework from a pyridine-containing heterocyclic Schiff base ligand. The 1H-NMR and 13C-NMR spectra of this zinc(II) complex, show the symmetric nature of the complex in which the Schiff base is coordinated to the zinc atom through the pyridine nitrogen, thus forming a head-on, N-Zn-N system. This is confirmed by the X-ray structure of the complex. The imine (-N=CH-) groups remain unusually uncoordinated.
Acknowledgements
The authors thank Dr. James Raftery of the University of Manchester for assistance with the single crystal x-ray structure determination.