1. Introduction
Digestive enzymes are vital for digestion and absorption of nutrients through breakdown of macromolecules such as proteins, carbohydrates and fats. Most of these digestive enzymes are hydrolases. They are secreted by various parts of the gastro intestinal tract (GI tract) [1]. These enzymes can either be enhanced or inhibited by binding with substrates that enhance or inhibit the enzymatic reactions. Inhibition of enzymes is inhibited by compounds with lower molecular weight also known as inhibitors. This inhibition process is also helpful in detoxification of the toxins produced and prevents the damage of GI tract due to over expression of the enzymes [2].
Inhibition alters the catalytic reactions of the enzymes that in turn could lead to deficiencies and related medical conditions in humans such as, G6PD deficiency, it leads to vulnerable breakdown of RBC, resulting in haemolytic anaemia, together with back pain, abdominal pain and jaundice. The symptoms usually associated with stressors or infections [3]. Goucher’s disease is due to the deficiency of enzyme pyruvate kinase, causes RBC breakdown leading to anaemia. Lactose intolerance is due to the deficiency of lactase enzyme. So, the consumption of lactose or milk products results in serious uncomfortable state [4]. To overcome the enzyme deficiencies, usually the patients are treated with Enhancer supplements from the plants and microbes which enhances the production of enzymes and ensures the enzyme substrate binding [5].
Apart from inhibition, enhancement of these catalytic reactions too leads to an array of medical conditions. Increased production of alpha amylase results in the increased rate of digestion of carbohydrates which may lead to increased blood glucose levels. Also over production of α-amylase (hyperamylasaemia) together with some co-factors such as consumption of alcohol leads to inflammation of pancreas, resulting in acute and chronic pancreatitis gradually leads to liver cirrhosis and hepatic carcinoma [6].
The over production of gastric juices such as pepsin triggers the production of HCl in the stomach as well as resulting in Gastro-oesophageal reflux. This acid formation also occurs due to Zolllinger-Ellison syndrome [7] where tumours present in pancreas or duodenum trigger the excess production of the gastric enzymes such as pepsin and HCl damaging the stomach and duodenum which may lead to gastric cancers or duodenal cancers. Enzyme inhibitors, therefore, could be potential strategies to avoid over production of enzymes or managing such medical conditions [8].
The aim of this research project is to investigate the inhibitory effects of Phyllanthus amarus leaves on digestive enzymes; α-amylase, trypsin and pepsin together with its thermo stability and ammonium sulphate precipitation of the enzymes.
1.1. Phyllanthus amarus and Its Medical Properties
Phyllanthus amarus also known as sleeping plant belongs to the family of Eurobiaccea which also has 800 varied species of it. It is mostly found in tropical and subtropical regions of the world [9]. and used in indigenous medicines like Ayurveda, siddha and Unani for treating conditions such as jaundice, bladder infections, ulcers, kidney disorders, diabetes, hepatitis B, cirrhosis and also urinary tract infections [10].
These medical conditions are treated with therapeutically designed supplements made up of Phyllanthus amarus plant leaves which is rich in some photochemical compounds such as alkaloids, saponins, tannins, glycosides, flavonoids, steroids and terpenes [11].
Phyllanthus amarus has a wide range of functions such as antiviral, anti-oxidant, hypolipidemic, anti-depressants, anti-inflammatory, antidiabetic, immunomodulatory and anti-cancer. These functions are clinically proved by the randomized trials in patients and also some on rats. Literature highlights these array of functions by P. amarus is due to the photochemical compounds which are rich in terpenoids, phenols, flavonoids, tannins, alkaloids, steroids and polypropanoids [11]. Some of the clinical trials are summarized in Table 1.
Apart from the inhibitory effect of this plant on digestive enzymes there were many studies on different pharmacological actions of this Phyllanthus amarus as shown in Table 1.
1.2. Importance and the Inhibitory Effects of P. amarus Leaves Extract on α-Amylase, Pepsin and Trypsin Enzymes
Starch is a fundamental source in human diet. Mammalian pancreatic and salivary
![]()
Table 1. Clinical trials on Phyllanthus amarus (P. niruri) leave extracts.
α-amylase hydrolyses α,1-4 glyosidic bonds through its endo catalytic activity. Starch is abundant with α,1-4 glyosidic bonds and a few α,1-6 glyosidic bond branches. This hydrolytic reaction plays a significant role in post prandial blood glucose level. Increased breakdown of starch leads to Diabetes type II (DM), metabolic syndrome and obesity [16].
DM II is mainly due to deficiency of insulin. Hallmark of treating DM is to control blood glucose level through hypoglycemic supplements which inhibits α-amylase activities. Since herbal supplements are acceptable, effective and safe. Herbs are preferred mostly to design drugs. Phyllanthus amarus plays a significant role in the inhibition of this enzyme α-amylase [17]. Inhibition of α-amylase by P. amarus was illustrated in Figure 1 below [18].
According to the study of Gao et al., 2008, experimental screening illustrates that methanolic extracts of Amaranthus species shows the highest amylase inhibitory effects compared with 40 other varied species of herbal plants. This plant is also promising to be an efficient supplement as the inhibitor of amylase enzyme.
Pepsinogen is a zymogen secreted by mucosal lining of the stomach. This secretion is controlled by vagus nerves and hormones such as gastrin and secretin. It becomes active as pepsin when mixed with HCl which provides an acidic, optimized environment for the enzyme. Pepsin through hydrolytic reaction breakdown peptide bonds of the protein in to amino acids and dipeptides. Overproduction of pepsin together with HCl give rise to gastric ulcers and gastric esophageal reflux. Hallmark of treating this condition mainly focus on the inhibition of pepsin enzyme [19].
Trypsin belongs to serine family of protease which is secreted by the pancreas as zymogen called trypsinogen. It is activated as trypsin by entero peptidase in
![]()
Figure 1. In a clinical trial inhibition of α-amylase by P. amarus and Acarbose (inhibitor drug) was compared using a spectrophotometer readings at 595 nm at different concentrations. 58.45% of inhibition of Acarbose at IC50 value of 83.33 ± 0.34 (μg/mL) and 75.32% of inhibition of P. amarus at IC50 of 48.92 ± 3.43 (μg/mL) as shown in Figure 2 [18].
the small intestine. Through hydrolytic reactions it digests proteins in to amino acids and dipeptides by breaking poly peptide bonds [20].
1.3. Importance of Ammonium Sulphate Fractionation and Thermal Stability
Therapeutic production of drugs highly focuses on the thermal stability of enzymes. Irreversible chemical and physical changes that occur when increasing the temperature is known as thermal stability [21].
Ammonium sulphate precipitation assay is done mainly to precipitate globular proteins or enzymes via salting-in procedure. Normally along with the increase of salt concentration stability of the protein known as salting out. Salting out is mainly based on the hydration layer where the protein is in contact with. There are 3 main protein water molecule interactions, Ion hydration between amino acids which are charged and mainly located in side chains (Asp, Lys), bond between polar residues located in main chains and water molecules (Tyr, Ser, Thr), bonds between apolar groups and water molecules. Precipitation occurs mainly by hofmeister series followed by the salts as shown in figure 5 [22].
2. Materials and Methods
2.1. Materials
2.1.1. Plant Materials
Leaves of Phyllanthus amarus which belongs to the family of Euphoria.
2.1.2. Chemicals
NaCl, KCl, NaH2PO4, 1% dimethyl sulfoxide, DNS reagents, hydrochloric acid (HCl), distilled water, hemoglobin powder, pepsin, starch powder, procrine α-amylase, methanol, 5% of trichloro acetic acid, phosphate buffer (pH 7.5), 1× phosphate buffer saline.
2.1.3. Equipment
Centrifuge tubes, Eppendorf tubes (1.5 ml), boiling tubes. Micropipettes (P-10, P-100, P-1000), Beaker, measuring cylinder of volumes 25 cm3, 50 cm3, 100 cm3, 250 cm3, Petridish, Falcon tubes (15 ml), water bath (Gemmyco), Centrifuge machine (80-2B), Electronic balance belongs to Scot pro, Autoclave, Fume hood (BIOBASE), Vortex machine, Electronic roller (KJMR-II).
2.2. Methods
2.2.1. Plant Material Preparation
Leaves of Phyllanthus amarus (P. niruri) were collected in the month of May from on specific district Trincomalee in Srilanka. Leaves were kept drying at room temperature and then dried leaves were crushed and ground in to a fine powder using an electronic grinder. The coarse powder was then stored at room temperature until the research procedures. All reagents were also prepared using standard protocols.
2.2.2. Hydro Methanolic Plant Extract
The coarse powder of Phyllanthus amarus (10 g) was extracted with 40 ml of 80% methanol by placing on roller at the room temperature (30˚C) for 48 hours. Sample was then filtered in to a Petri dish and incubated in the fume hood for 24 hours until the methanol completely evaporated. The sediment left on the Petri dish was then scraped off using a surgical blade and stored into an Eppendorf tube. Then the sample weight was measured using an electronic balance and stored at room temperature until the research assays were started.
2.2.3. Hydro Methanolic Plant Extract
40 mg/ml stocks were prepared using 1% DMSO. From the stock working solutions were prepared again using 1% DMSO.
2.3. Methodology for Assays
2.3.1. Pepsin Inhibition Assay
100 µg pepsin, 1600 µg hemoglobin (Hb) and extracted sample at concentrations of 0.04 mg/ml, 0.08 mg/ml, 0.16 mg/ml, 0.32 mg/ml, 0.64 mg/ml and 1.28 mg/ml were mixed in to 1000 µl of reaction mixture. The reaction mixture was incubated at 37˚C for a period of 20 minutes. After incubation 700 µl of 5% TCA was added to stop the reaction, and centrifuged at 4000 rpm for 20 minutes. Then the supernatant was taken out in to the cuvette without disturbing the pellet. And the absorbance reading was taken in the wavelength of 240 nm. No plant extract was added to the control and no enzyme was added to the blank [23]. The assay was repeated three times.
2.3.2. α-Amylase Inhibition Assay
200 µg α-amylase and extracted sample at concentrations of 0.04mg/ml, 0.08 mg/ml, 0.16 mg/ml, 0.32 mg/ml, 0.64 mg/ml and 1.28 mg/ml were mixed in to 800 µl of reaction mixture. The reaction mixture was incubated at 25˚C for a period of 10 minutes. Then starch (1 mg) was added to 800 µl of the reaction mixture and incubated at 25˚C for a period of 10 minutes. To stop the reaction 400 µl of 3,5-DNS solution (96 mM) was added to the reaction mixture, and incubated at the temperature of 99˚C for a period of 5 minutes. Absorbance reading was taken in the wavelength of 540 nm. No plant extract was added to the control and no enzyme was added to the blank. The assay was repeated three times [24].
2.3.3. Trypsin Inhibition Assay
5 µg trypsin, 2 ml of casein (10 mg in the form of fresh Ambewala milk packet of 0.005 g/ml), sodium acetate buffer of 150 µl and and extracted sample at concentrations of 0.04 mg/ml, 0.08 mg/ml, 0.16 mg/ml, 0.32 mg/ml, 0.64 mg/ml and 1.28 mg/ml were mixed in to 3000 µl of reaction mixture. Then the reaction mixture was incubated at the room temperature for a period of 10 minutes. To stop the reaction 4 ml of 5% TCA was added to the reaction mixture, and centrifuged at 4000 rpm for a period of 20 minutes. Then the supernatant was taken out and filtered using a watman filter paper in to the cuvette and the absorbance reading was taken in the wavelength of 320 nm. No plant extract was added to the control and no enzyme was added to the blank. The assay was repeated three times [25].
2.3.4. Thermal Stability Assay
The plant extracts were incubated at room temperature of 29˚C, 4˚C, 37˚C, 60˚C, 80˚C and 99˚C separately for a period of 30 minutes. These pre-incubated plant extracts were used for all 3 inhibitory assays (Pepsin, Trypsin and Amylase) with the same procedures as mentioned above. These assays were repeated three times. The concentration of the preincubated plant extract were equal (1.28 mg/ml).
2.3.5. Inhibitory Assays on Ammonium Sulphate Precipitation Assay
Ammonium sulphate solutions were prepared at 3 different concentration gradients of 30%, 60% and 90% of w/v% respectively. Plant extracts of the concentration of 4 mg/ml was then added to obtain 15%, 30% and 45% w/v% of ammonium sulphate solutions respectively. These solutions were incubated in the ice for 10 minutes, and centrifuged at 4000 rpm for a period of 20 minutes. Then the supernatant was poured out, remaining pellet was dissolved in 2 ml of 1× phosphate buffer saline (PBS). This dissolved solution was then used as protein fraction and the 3 assays were proceeded and repeated 3 times.
3. Results and Discussion
Percentage of inhibition for all three inhibitory (pepsin, trypsin and amylase) assays were examined. The results obtained were analyzed statistically using ANOVA. The concentrations are not at equal intervals and since the concentrations were at two folds the log base 2 graph was obtained as it will give a better idea of the percentage of inhibition of the enzymes inhibitory assays with a uniform interval of different concentrations.
3.1. Results for the Inhibition Assays
According to Graphs 1-3 the potential inhibition of trypsin enzyme by Phyllanthus amarus was determined. The P value for this inhibition assay was P = 0.005 (P < 0.05). Although there was a slight deviation observed there is a significant relationship between the concentration of the plant extract and the inhibition of trypsin enzyme. The rate of inhibition of the trypsin enzyme significantly increased with increasing concentration of the extract. However maximal inhibitory effect was exhibited at the concentration of 1.28 mg/ml and minimal inhibition was observed at the concentration of 0.16 mg/ml.
3.2. Results for the Thermal Stability Assays of the Three Enzymes
The inhibitory effect of plant extract on enzymes together with different temperatures were examined. According to the statistical analysis of ANOVA the P
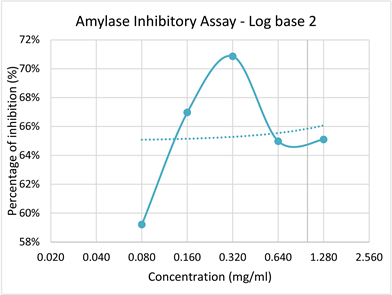
Graph 1. Log base 2 graph of Percentage inhibition of α-amylase with increasing concentration of plant extract.

Graph 2. Log base 2 graph of Percentage inhibition of pepsin with increasing concentration of plant extract.
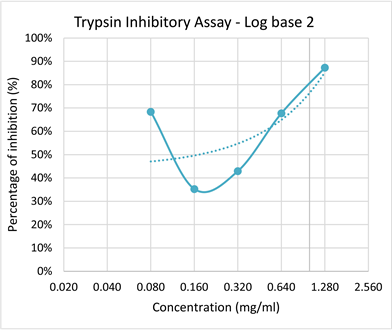
Graph 3. Log base 2 graph of Percentage inhibition of Trypsin with increasing concentration of plant extract.
value for amylase enzyme was determined as P < 0.05, P = 0.0000089. which indicates that there is a strong significant relationship between the activity of the enzyme and the temperatures at which the extract were incubated. It was observed that with increasing temperature the enzyme activity also increased apart from minor fluctuations according to the absorbance reading obtained, the highest inhibitory rate was observed at the temperature of 80˚C and the lowest inhibitory effect was observed at the temperature of 60˚C as shown in Graph 4.
Whereas the P value for pepsin thermal stability was determined as P > 0.05, P = 0.059. which indicates that there is no strong significant relationship between the activity of the enzyme and the temperatures at which the extract were incubated. It was observed that with increasing temperature, the enzyme activity was decreased according to the absorbance reading obtained. Highest inhibitory effect was observed at 4˚C and lowest inhibitory effect was observed at room temperature (29˚C) as shown in Graph 5.
According to Graph 6 the P value for trypsin thermal stability was determined as P < 0.05, P = 0.00000142. Which indicates that there is a strong significant relationship between the activity of the enzyme and the temperatures at which the extract were incubated. It was observed that with increasing temperature the enzyme activity was decreased according to the absorbance reading obtained.
3.3. Enzyme Inhibitory Assays on Ammonium Sulphate Precipitation
ANOVA statistical analysis was done for 15%, 30% and 45% of ammonium sulphate fractions with the plant extracts of concentration 4 mg/ml for all three assays. The P values determined for 15%, 30% and 45% of ammonium sulphate precipitation in all three assays. When P < 0.05 this indicates that there is a significant relationship between the concentration of ammonium sulphate and the protein fractions obtained from them. When P > 0.05 this indicates that there is no significant relationship between the concentration of ammonium sulphate and the protein fractions obtained from them. In here, highest inhibition was

Graph 4. The change in absorbance with increasing temperature reflecting the reduced enhancing potential of plant extract with increasing temperature on amylase.

Graph 5. The change in absorbance with increasing temperature reflecting the reduced inhibition potential of plant extract with increasing temperature on pepsin.
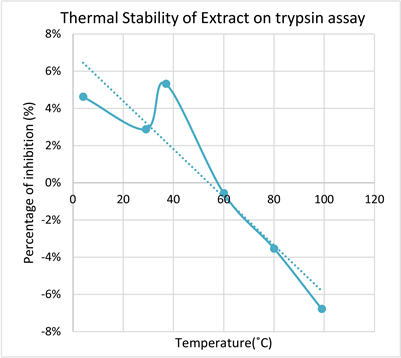
Graph 6. The change in absorbance with increasing temperature reflecting the reduced inhibition potential of plant extract with increasing temperature on trypsin.
observed in plant extract of 4 mg/ml at 30% of ammonium sulphate fraction for pepsin and trypsin inhibition assays and in 45% of ammonium sulphate fraction for α-amylase assay. The percentage of ammonium sulphate fraction at which the inhibition was maximum is shown in Table 2.
4. Discussion
This research was to examine the inhibitory effects of medical herb Phyllanthus amarus on α-amylase, pepsin and trypsin enzymes [26]. As mentioned before chemical compounds known as polyphenols present in P. amarus leaves were
![]()
Table 2. % of ammonium sulphate fraction at which the inhibition was maximum for 4 mg/ml concentration of the plant extract.
responsible for the inhibitory effect on these digestive enzymes [27]. As mentioned earlier since diabetes mellitus type II is one of the chronic disease challenging today’s world, management of it is highly focused on reducing post prandial blood sugar level through inhibition of enteric enzymes such as α-amylase and α-glucosidase [11]. Therapeutic approach mainly targets on these medical herbs and plant extracts since they are harmless, effective and efficient [28].
A study conducted by Lawson-Evi et al., 2011, clearly depicts the inhibitory nature of P. amarus leave extracts. In this study, alloxan induced diabetic rats were treated with hydro alcoholic P. amarus leave extracts, aqueous extracts of P. amarus leaves and some of them left untreated. Both the groups of rats treated with P. amarus leaves extract showed significant decrease in blood glucose level and body weight. Along with regeneration of β cells and high levels of insulin secretion thereby supporting the principles that these P. amarus leaves have antidiabetic (inhibitory effect on α amylase) and antioxidant properties which will help to manage diabetes mellitus type II [29].
In this research although there were little fluctuation in the rate of inhibition, overall it elicits a high inhibitory potential of P. amarus methanolic extract with increasing concentration. The maximal inhibitory effect for amylase enzyme was observed at the concentration of 0.32 mg/ml with the P value of 0.0855 (P > 0.05). these results also were supported by scientific research works as well. However, the methodology adapted indicated that the plant extract exhibited inhibition of α-amylase at 0.5 mg/ml. But in here, the rate of inhibition increased with the increasing concentration of P. amarus extract [30]. Apart from experimental errors 0.32 mg/ml showed maximal percentage of inhibition of 71%.
This might be due to the increasing concentration provides more active components of the P. amarus extracts to inhibit the enzyme α-amylase. That’s what lower concentration 0.08 mg/ml of the extract showed moderate inhibition of 59% due to its moderate chemical compounds available for inhibition. Meanwhile 0.32 mg/ml also provides more number of active sites available thus eliciting an effective inhibition nature of P. amarus.
This inhibition value was somewhat consistent with another study conducted on methanolic extracts of Phyllanthus amarus leaves by Oyewole, Taiwo and Quadri (2014), showed around 79% of inhibition of α-amylase enzyme. Since there is decreased levels of percentage of inhibition in concentration 0.64 mg/ml and 1.28 mg/ml than in 0.32 mg/ml, the 0.32 mg/ml concentration could be considered as the maximum efficient concentration of this plant extract for the inhibition of α-amylase enzyme in this assay [30].
Literature show cases that photochemical compounds present in P. amarus extracts such as Tannins, Lignans, Ellagitannis, Sapannins and Tritepene are highly responsible for hypoglycemic effect, thereby used as therapeutics to heal Diabetes mellitus [16].
In pepsin assay the maximum inhibition was noticed at the concentration of 0.08 mg/ml along with 85% of the percentage of inhibition. However the experimental errors has brought unexpected values for inhibition at some concentrations of the extract, overall inhibition percentage illustrates that the P. amarus leave extracts has inhibitory effects on pepsin enzyme as well. In this study, for the pepsin assay hemoglobin was used as substrate since hemoglobin is also a type of globular protein. So that pepsin breakdown hemoglobin this enzymatic reaction was inhibited by P. amarus methanollic leave extract.
This inhibition value was somewhat consistent with a similar study conducted on alcoholic extracts of Phyllanthus amarus leaves by Abdulla and his colleagues (2010), showed around 81% of inhibition of pepsin enzyme [31].
According to certain studies they reveal Flavinoids, Glycosides, Tannins and Lignans in P. amarus methanolic extracts are responsible for the anti-oxidant property which is also used to inhibit the pepsin enzyme and heal gastric ulcers [32].
Literature also supports the inhibitory effect of P. amarus on pepsin enzyme. Even in a study conducted on Indomethacin induced Albino rats with the weight of 150 - 200 g, were orally administered with methanolic extracts of P. amarus leaves and some left untreated [32]. The treated rats showed P < 0.05 significant inhibition of pepsin enzyme and recovery from Indomethacin induced gastric ulcers. Which supports, the assay results. Although along with 5 different concentration of the extract the percentage of inhibition is decreasing. The chemical compounds found in P. amarus leaves highly supports that this has inhibitory effects on the pepsin enzyme [33].
In trypsin assay, according to the ANOVA statistical analysis showed P < 0.05 (P = 0.005) which shows a significant relationship between the percentage of inhibition with increasing concentration of the plant extract. the high concentration of 1.28 mg/ml of P. amarus extract showed high percentage of inhibition of 87% to trypsin enzyme.
Moreover, Phyllanthus amarus methanolic extracts shows inhibitory effects on these three digestive enzymes such as pepsin, amylase and trypsin. Figurebelow shows the comparison of inhibitory effects of P. amarus on these 3 enzymes along with their concentrations as shown in Graph 7.
As shown in Graph 8 the thermal stability assay was conducted to evaluate
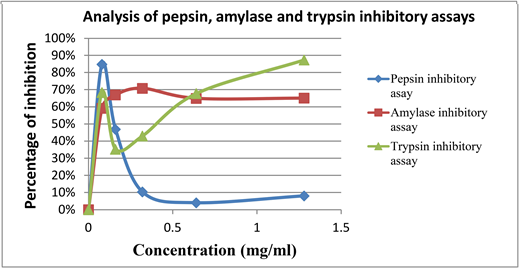
Graph 7. Comparison of pepsin, trypsin and amylase inhibitory assays.

Graph 8. Comparison of thermal stability on pepsin, trypsin and amylase inhibitory assays.
the protein constituents in P. amarus methanolic extract. Also to determine its impact on the pepsin, amylase and trypsin inhibitory assays. ANOVA statistical analysis showed amylase (P < 0.05, P = 0.0000089) and trypsin (P < 0.05, P = 0.0000142) inhibitory assays showed a significant relationship between the thermal stability and the inhibitory effects of the respective enzyme whereas, pepsin inhibitory assay (P > 0.05, P = 0.059) doesn’t show a significant relationship between thermal stability and inhibitory effect. Apart from the experimental errors rate on inhibition and thermal stability is high for Amylase >Trypsin > Pepsin respectively.
The literature also supports that pepsin inhibition of the P. amarus extract was less along with increasing temperature is because since flavonoids are the main constituent in the extract which enhances the inhibitory rate of pepsin enzyme, these flavonoids showed higher degradation rate along with temperature rise [7]. That’s what the rate of inhibition does not show a significant relationship.
According to Graph 9 the ammonium sulphate precipitation assay was done to purify the proteins in the P. amarus extract and to validate the impact of it in the protein, trypsin and amylase inhibitory assays. Whereas, only amylase P < 0.05 which showed a significant relationship between ammonium sulphate
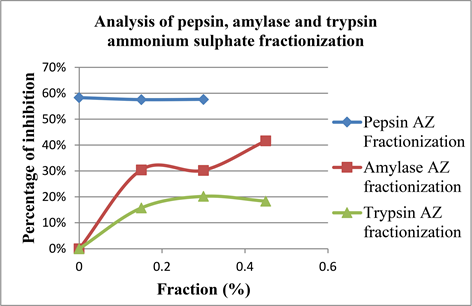
Graph 9. Comparison of ammonium sulphate precipitation on pepsin, trypsin and amylase inhibitory assays.
precipitation and the percentage of inhibition. The percentage of inhibition was high at 50% fractionate of ammonium sulphate whereas, for pepsin and trypsin the P values were P > 0.05 (P = 0.0005).
Apart from the experimental errors studies suggests that due to reversible inhibition of enzymes at the fractioning rate might interfere with the rate of inhibition and also it suggests the pH should be maintained constant along the whole procedure [34].
In a study on P. amarus ammonium sulphate solid was directly added to the plant extract to get 82% of saturation at the pH of 7.1 [35], where the percentage of pepsin inhibition was significantly high which shows that higher the saturation percentage higher the protein purification.Also in enzyme analysis ammonium sulphate fractionation play a crucial in the evaluation of specific and total activities of enzymes [36].
From the research it was well understood that photochemical compounds and polyphenols present in Phyllanthus amarus are the major key players in the inhibition of several enzymes including pepsin, trypsin and amylase. Antioxidant properties also supports the plant to be the leading choice of interest in therapeutics and indigenous medicine. It is also evident these active constituents in P. amarus could be effected by temperature, pH and concentrations.
A systemic clinical analysis on the therapeutic effects of P. amarus was done by [37]. In this study, the fractioning and analysis of the components of P. amarus was examine which showed Tannins acts as the most inhibitor towards all enzymes and also play a vital role as an anti-oxidant which recently recovered tumorigenesis in rats diagnosed with hepatic cancer and treated with aqueous P. amarus extract [37].
Moreover, Phyllanthus amarus could be suggestive for a potential use of Herb-drug interaction and supplementation to cure so many disease conditions such as Diabetes mellitus, Gastric ulcers which result due to overproduction of digestive enzymes and also it could be treated for Jaundice, inflammatory conditions, infections and also cancer for its inhibitory affect and antioxidant effect due its photochemical compounds and polyphenols [29].
The results might vary because of experimental errors. The possible errors that might occur in the practical process as shown in Table 3.
Future Works
Although the research revealed polyphenols are playing a major role in the inhibitory effect of the extract, these constituents can be individually separated by high performance liquid chromatography (HPLC) technique so that specific analysis on inhibition will be accurate.
In vivo analysis of the results obtained should be done to ensure the same effectiveness is possible within the human system if applied to rule out further complications to use them as supplements in therapy. Although these extracts are highly appreciable in therapeutics and medicine, side effects or the health issues though their supplements also should be tested.
Ammonium sulphate fractioning could be purified using analytical techniques such as HPLC, gel filtration chromatography and ion exchange chromatography and tested specifically with each active component present in the extract.
Although the components of the plant extract show inhibitory effects towards Pepsin, Trypsin and Amylase enzymes, analysis should be done further to evaluate the period of inhibition and its effectiveness.
Since antioxidant property of the plant extract enhances the inhibitory effect
![]()
Table 3. Experimental errors and the relevant precautionary methods [36] [38].
specifically inhibiting pepsin and healing gastric ulcers, inhibitory assays should be done on lipase enzyme to check for the connection or the antioxidant nature and the components responsible for it. Since Phyllanthus amarus play a crucial role in the treatment of jaundice and inflammations. In here milk is used instead of trypsin. A proper method should be followed to dissolve the trypsin powder available in the laboratory to ensure that trypsin without any other constituents which can affect the enzyme activity during the assay.