Strain-rate cycling tests associated with the ultrasonic oscillation were conducted for the purpose of investigation on the interaction between dislocation and I
– ions during plastic deformation of NaCl:I
– (0.5 mol% in the melt) at 77 K to room temperature. The relative curves of stress decrement (
Δt) due to the oscillation and strain-rate sensitivity (
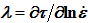
) have stair-like shape for NaCl single crystals doped with I
– at low temperatures. There are two bending points and two plateau regions.
λ decreases with
Δt between the two bending points. τp at
Δt of first bending point and
λp between
λ at first plateau place and at second one depend on the dopant ions as weak obstacles to dislocation motion. Not only temperature dependence of
τp and
λp but also
τp versus
V (activation volume) reflects the interaction between dislocation and I
– ions. On the basis of the data (
i.e. τp and
λp) analyzed in terms of the relative curves of
Δt and
λ, the activation energy,
G0, for the overcoming of dislocation from the dopant ion is found to be 0.47 and 0.53 eV for NaCl:Br
– and NaCl:I
–, respectively. This result that
G0 for NaCl:I
– is somewhat larger than for NaCl:Br
– leads to the phenomenon that I
– ions are slightly stronger than Br
– ones as weak obstacles to dislocation motion because of the difference between isotropic strains around I
– ion and around Br
– in NaCl single crystal. Furthermore, the values of
τp0 and
Tc are also obtained for the two kinds of specimens.
τp0 and
Tc are the value of
τp at absolute zero and critical temperature at which
τp becomes zero.