Ruthenium complexes present two states of oxidation that are Ru(II) and Ru(III). Both are assumed to present cytotoxic activity at ground state. On the purpose of highlighting their differences, DFT, TD-DFT and NBO have been performed at both Wb97xd/Lanl2dz and B3lyp/Lanl2dz levels. NBO program shows that both groups of ruthenium complexes present almost the same charge of Ru atom. Moreover, they display nearly the same structure of valence orbitals of the ruthenium. However, when it comes to compare their frontier orbitals HOMO and LUMO, we notice that the chloride atom has a great influence on their energy. The lack of Chloride atoms reduces the energy of frontier orbitals regardless of the functional. And the more the number of chloride atoms, the higher the energy. Also, RuCl
3Terpy and α-RuCl
2(Azpy)
2 have been discovered to display the best energy suitable for reaction as cytotoxic agents. Yet, both are from groups different. Thus, at ground state, there is practically no difference between both groups. However, regarding TDDFT prediction with the determination of vertical electronic affinity VEA and vertical ionization potential VIP both at ground state S and at exciting T1 state, we notice that Ru(II) complexes are not active either in the presence or absence of
3O
2 molecule. Here, only Ru(III) complexes are able to react on Guanine through their radical cations or by generating the superoxide radical anion
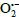
. Therefore, the Ru(III) complexes are assumed to be active both at a fundamental state and under the effect of light for photodynamic therapy. We come to conclude that Ru(II) complexes are not active by excitation as their valence electrons are paired thereby making these complexes more stable. Besides,
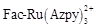
, a Ru(II) molecule that is not active at ground state owing certainly to its C
3 symmetry or Azpy ligand presents all the same a difficult activity on generating
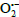
. For the coming paper, we intend to check whether Ru(II) complex can be active under the effect of light if it is in a triplet charge state.