Calculations of chemical structures and photofading of parabens (PHB—4 hydroxybenzoic acid), which are p-hydroxybenzoic acid alkyl esters were performed. These compounds are used as preservatives for the substances used in cosmetics. The reactivity of these derivatives with an oxidant—singlet oxygen—have been tested with a theoretical method of frontier orbitals. All-valence molecular orbital methods, AM1 and PM3, have been used to calculate frontier electron density for higher occupied HOMO and lower unoccupied LUMO orbitals, which might be sensitive to an electrophilic (with singleton oxygen atom
1O
2) or nucleophilic (
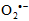
superoxide anion radical) attack at a particular atom in a molecule. Using AM1 and PM3, we calculated the reactivity
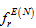
, superdelocalisability
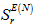
and electron density distributions. The obtained superdelocalisability rates allow you to explain the fastness values in different chemical molecules. The structure of parabens (PHB) was optimized by MM+, DM, AM1 or PM3, to achieve constant energy values at a convergence criterion of 0.01 kcal/mol. The performed calculations indicate that the electrophilic oxidation reaction should take place in the aromatic ring in the 2-position to the hydroxyl residue of PHB, whereas the superoxide radical reaction occurs mainly on the alkyl residues of the ester group. The reaction may take place according to superoxide mechanism or 1,2-addition, where the higher superdelocalisability values SN are located on neighboring atoms in aromatic systems.