The kinetics and stoichiometry of the reduction of H
2O
2 by an aminocaboxylactocobaltate(II) complex (hereafter[CoHEDTAOH
2]
-) in aqueous medium have been studied under the following conditions: T = 29℃ ± 1℃, Ionic Strength, I = 0.50 mol dm
-3 (NaClO
4), [H
+] = 1 × 10
-3 mol dm
-3. The ratio from the stoichiometric study conforms to the equation 2[CoHEDTAOH
2]
- + H
2O
2 + 2H
+ → 2 [CoHEDTAOH
2] + 2H
2O. The rate of reaction varied linearly to the first power of the concentrations of the reductant and oxidant and displayed inverse dependence on acid concentration. The plot of acid dependent rate constant versus [H
+]
-1 was linear with zero intercept. The [CoHEDTAOH
2]
- - H
2O
2 reaction was insensitive to the change in ionic strength of the medium suggesting interaction of charged and uncharged species at the activated complex. The Michaelis-Menten plot of
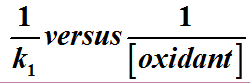
was linear without intercept which suggested absence of intermediate complex. Evidences in this paper showed that the reaction occurred through the outer-sphere mechanism.