The first hydrolysis constants of trivalent holmium in 2 M NaClO
4 and 2 M NaCl at 303 K and in CO
2 free conditions were determined. The pC
H borderlines of precipitation and first hydrolysis were determined by means of a spectrophotometric method and last one with the program SQUAD. Independently, the stability constant for the first hydrolytic species was determined, by means of potentiometric pH titrations whose data were treated with the program SUPERQUAD. The hydrolysis constants obtained were:
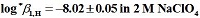
and

. These values attained by both methods are the same. The log
10β1,Cl constants for the species HoCl
2+ was also calculated for 2 M ionic strength and 303 K from the hydrolysis constant obtained in both perchlorate and chloride media. This value was log
β1,Cl = -0.56.