The crystallization in the
three-component systems Rb2SO4-MSO4-H2O (M = Mg, Co, Ni, Cu,
Zn) is studied by the method of isothermal decrease of supersaturation. It has
been established that isostructural double compounds,
Rb2M(SO4)2·6H2O (M = Mg, Co, Ni, Cu, Zn),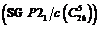 , crystallize from the
ternary solutions within wide
, crystallize from the
ternary solutions within wide
concentration ranges. The infrared spectra are discussed with respect to
the normal vibrations of the sulfate ions and water molecules. The unit-cell
group theoretical treatment of the double salts is presented. The extent of
energetic distortions
of 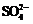 guest ions (about 2 mol%) matrix-isolated in
the respective selenates,
guest ions (about 2 mol%) matrix-isolated in
the respective selenates, 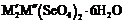 (M' =
K, Rb,
(M' =
K, Rb,  ; M" = Mg, Co, Ni, Cu, Zn), is commented.
; M" = Mg, Co, Ni, Cu, Zn), is commented.