1. Introduction
Skin cancer incidence is increasing at alarming rates. In the US alone, more than two million people develop over 3.5 million nonmelanoma skin cancers every year. This translates to more than 300 percent increase in cancer incidence since 1992 [1,2]. This observation does not only apply to the US, but is a worldwide phenomenon. Skin cancer incidence is considered by some as an epidemic and its incidence is higher than all other cancers combined.
Nonmelanoma skin cancers, such as basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), are the most common forms of skin cancer. Malignant melanomas are the least common but most serious type of skin cancer. Although malignant melanomas only constitute less than 5 percent of the incidences of all skin cancers, over the past 30 years incidences rates of malignant melanoma in Britain alone, have increased more rapidly than any of the top ten cancers in males and females. Increased rates of over 500 percent have been reported for malignant melanoma from 1975 to 2008 [3].
A variety of treatments are available for nonmelanoma skin cancers with good outcomes, especially if the cancers are detected and treated in the early stages of development. However, there are some serious disadvantages with the most common treatments. Some disadvantages of current treatments [2] include:
• margin around cancer may not be free of cancer;
• moderately painful;
• slow healing;
• scarring;
• specialized training by health professionals with appropriate facilities;
• expensive;
• activity restriction after surgery if skin graft or flap is needed;
• limited cosmetic results often with disfigurement;
• treatments are nonspecific, they do not distinguish between the killing and removal of cancerous tissues and the killing and removal of normal tissues.
In addition high recurrence rates of treated skin cancers have been reported [4].
Each Currently Used Skin Cancer Treatment Follows Specific Procedures
Surgical Excision. This surgical procedure is used to treat primary and recurrent tumours under local anesthesia. The tumour and an area of healthy looking skin (margin) around the tumour are removed surgically. The resulting wound is usually closed with stitches. Often, skin from another area of the body (skin graft), or healthy skin moved from a nearby area (skin flap) is used to complete the treatment. After surgery, the excised tissue is examined under a microscope to see if any cancer cells were present in the skin that appeared cancer free. The cure rates range from good to bad [4]. A limitation of this method is that this procedure does not distinguish between tumours and normal skin. The major shortcoming of surgical excision is the pain and discomfort and the potential for long term scarring and quality of life. This method of treatment is by its nature invasive, and at times disfiguring.
Mohs Micrographic Surgery. Mohs surgery has the highest reported cure rate for both basal cell and squamous cell carcinoma. This specialised surgical technique removes the visible tumour first and then removal of successive layers of skin one at a time until microscopic examination no longer reveals cancer cells. Mohs surgery is carried out while the patient is under local anesthesia. Removing and examining each layer takes a long time, usually over one hour. Once skin cancer is no longer visible under the microscope, the surgical wound is treated as needed and varies from stitches to skin flap. This treatment is invasive, and at times disfiguring. Mohs surgery is a treatment for most nonmelanoma skin cancers. However, the length and intensity of this surgical procedure limit its use to treating recurrent skin cancer, larger tumours, areas where it is essential to preserve as much skin as possible (such as an ear, eyelid, nose, lip or hand), tumours in which it is difficult to establish when the cancer ends, and sites prone to recurrence. This surgery when in process does not distinguish cancer cells from normal cells.
Curettage and Electrodesiccation. This method is used to treat small basal cell and squamous cell carcinomas in non-crucial areas such as the trunk and extremities.
The procedure consists of scooping out the cancer by using a spoon-like instrument called a curette and then using an electric needle to burn or “cauterize” the remaining cancer cells and to control bleeding. The scraping and cauterizing is typically repeated 3 times, and the wound tends to heal without stitches. This procedure does not distinguish cancer cells from normal cells.
Cryotherapy. Cryotherapy is a treatment in which surface skin lesions are frozen. Liquid nitrogen is the most common method used to freeze skin lesions. Cryotherapy also known as cryosurgery is mostly used to treat solar keratoses. However, it is sometimes also used to freeze small skin cancers such as superficial basal cell and in situ superficial squamous cell carcinomas (Bowen’s disease), but this is not always successful and careful follow-up is necessary. Cryotherapy stings and may be painful. There may be immediate swelling and redness. The treated area is likely to blister within a few hours. Within a few days a scab forms and the blister gradually dries up. The blister dries to a scab. The scab peels off after 5 - 10 days on the face and 3 weeks on the hand. A sore or scab may persist as long as 3 months on the lower leg because healing at this site is often slow. Cryotherapy may result in a white mark (hypopigmentation) or a scar, particularly when freezing has been deep or prolonged, as is required for cancerous lesions. Skin lesions may fail to clear or may recur at a later date, necessitating further cryotherapy, surgery or other treatment. Cryotherapy does not distinguish between normal or abnormal skin. Whatever area on the skin the liquid nitrogen (temperature –196˚C) touches, it kills.
Radiation Therapy. Radiation treatments for skin cancer are used in areas that are difficult to treat with surgery. It is used for large tumours, tumours that cover a large area, or tumours that are difficult to surgically remove because of location, such as eyelids, ears or noses. To obtain a good end result, the procedure involves many treatment sessions, usually 25 - 30. This procedure does not distinguish tumours from normal tissue.
Laser Therapy. Laser therapy may be used to vaporize superficial and multiple basal cell carcinomas and to excise or destroy squamous cell carcinoma. This therapy does not destroy cancer cells found deeper in the skin, so close follow-up with the patient is essential. This therapy does not distinguish tumours from normal tissue.
Topical Cream Treatment. Medical therapy using creams that contain anticancer agents (5-fluorouracil, 5-FU Efudex, Fluoroplex) or stimulate the immune system (imiquimod) are used to treat skin lesions. These creams are applied several times a week for several weeks. They produce brisk inflammation and irritation. Their limitations include discomfort, with many side effects, which may be severe, and a low cure rate, which makes medical treatment unsuitable for treating most skin cancers on the face.
Currently, surgical excision is the most common form of treatment for skin cancers. The goal of reconstructive surgery is restoration of normal appearance and function. The choice of technique in reconstruction is dictated by the size and location of the defect. Excision and reconstruction of facial cancers is generally more challenging due to the presence of highly visible and functional anatomic structures in the face.
The treatment and management of nonmelanoma skin cancers cost the USA health care system more than US $1.4 billion per year and this value is increasing dramatically each year. In the current world-wide economic situation this poses a financial burden and because of this, many patients afflicted with skin cancers may not seek proper treatment resulting in increased morbidity and mortality [2].
Unfortunately, the developments of new treatments for skin cancers have not parallelled the increased incidences of skin cancers.
Over the last few years a new treatment for nonmelanoma skin cancers has been described in the scientific literature. Any proposed new treatment for whatever purpose, must be at least as good or preferably better than current treatments.
This review examines whether the evidence of this new treatment lives up to its claims. The purportedly new treatment is a topical cream, CuradermBEC5, that is used to treat nonmelanoma skin cancers.
Treatment of nonmelanoma skin cancers with CuradermBEC5 claims the following:
• is derived from plant extracts;
• kills cancer cells by an unique procedure;
• kills cancer cells whether they are resting or multiplying, ensuring that all cancer cells are eliminated rapidly;
• at therapeutic doses kill cancer cells only and not normal cells;
• is more effective and safer than other well established anti-cancer drugs;
• is non invasive by simple application of a cream;
• shows superior cosmetic outcomes compared with other available treatments.
2. Discussion
What evidence is available to justify the claims?
2.1. The Therapeutic Agents in CuradermBEC5 are Derived from Plant Extracts
In 1987 it was reported that a standard mixture of glycoalkaloids (BEC) was extractable from the fruit of Solanum sodomaeum also known as S. Linnaeanum (Devil’s Apple). BEC is a mixture of solasodine glycosides consisting of the triglycosides solasonine (β-solatriose), solamargine (β-chacotriose), and di and monoglycosides. All the glycosides contain the same aglycone, solasodine [5-9].
Figure 1 shows the chemical structures of the two main glycoalkaloids, solasonine and solamargine [5-8] and figure 2 shows their mass spectra [9].
It was subsequently shown that the identical replica of BEC is also present in the eggplant or aubergine [10].
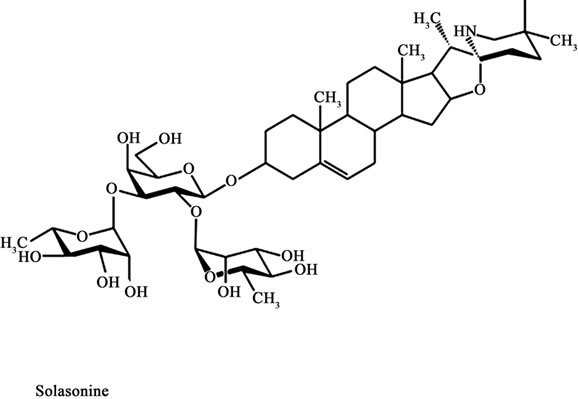
Figure 1. Chemical structures of solasonine and solamargine.

Figure 2. Mass spectra of solasonine and solamargine.
2.2. The Therapeutic Agents in CuradermBEC5 Kill Cancer Cells by an Unique Procedure
BEC, at the appropriate dose, only interacts with cancer cells and not with normal cells [6,11-28].
A specific protein has been identified on cancer cells. This protein, known as rhamnose binding protein, is not, or very limited, on normal cells [22]. The rhamnose binding protein is a receptor on cancer cells and binds BEC. After internalization in the cancer cell by receptormediated endocytosis through “coated pit endocytosis” a receptorsome or endosome is formed. Gradual transformation of endosomes results in the formation of lysosomes [29]. BEC then triggers extrinsic and intrinsic apoptotic pathways in the cancer cells by up-regulating the expressions of external death receptors, such as tumour necrosis factor receptor 1 (TNFR-1), Fas receptor, TNFR-1 associated death domain and Fas-associated death domain. BEC enhances the intrinsic ratio of Bax to Bcl-2 by up-regulating Bax and down-regulating Bcl-2 and Bcl-xL expressions. These effects result in activation of Caspase -8, -9 and -3 in cancer cells, indicating that BEC triggers extrinsic and intrinsic apoptotic pathways in cancer cells [6,30-37].
Apoptosis or programmed cell death is a highly organized physiological process that results in the removal of unwanted cells. Induction of apoptosis in cancer cells or malignant tissues is accepted as an efficient approach for cancer chemotherapy.
Figure 3 shows that BEC rapidly kills cancer cells by the characteristic apoptotic pathway of cell shrinkage, condensation of chromatin and nuclear fragmentation [32,38].
A recent study has shown that solamargine, the main component of BEC, also kills cancer cells by oncosis. After interaction of solamargine with cancer cells, marked changes in cell shape and volume occur. The cells get blebs on the membrane, the mitochondria swell, the contents of the nuclei clump and the cells die. It has been proposed that apoptosis and oncosis share certain mechanisms and alterations within the cell before they die by bursting. A study has shown that solamargine at low concentrations kill cancer cells by apoptosis and at higher doses solamargine kill cancer cells by oncosis, and both types of cell death are induced by intermediate concentrations of solamargine [39].
 (a)
(a)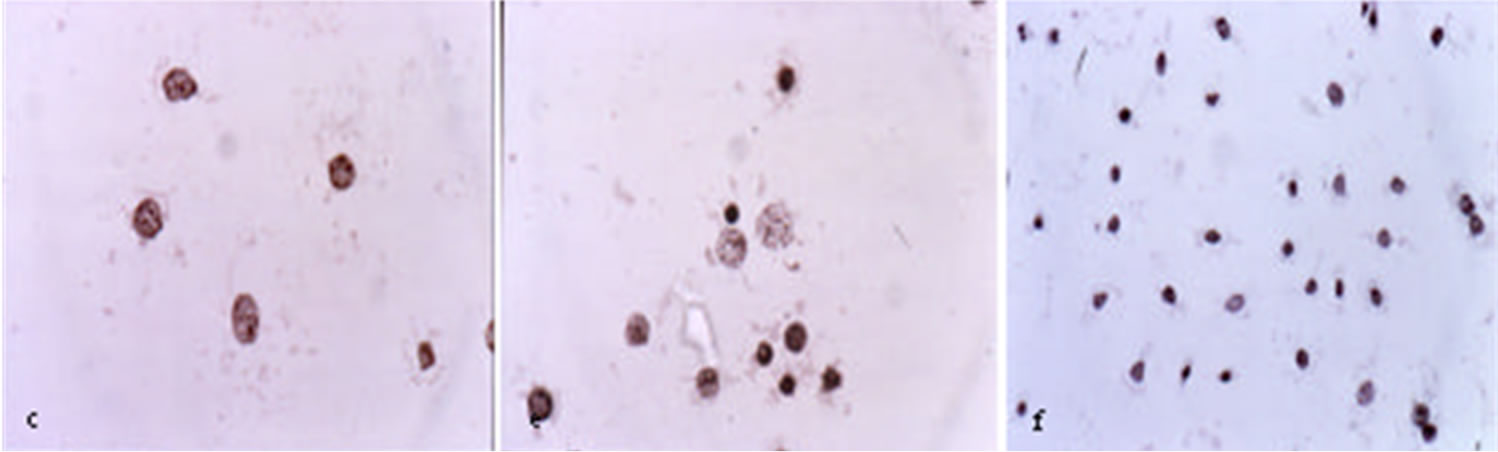 (b)
(b)
Figure 3. Untreated ovarian cancer cells, the cells are all viable (a) BEC causes the cytoplasm of the cancer cells to undergo dissolution, the nuclei contract and become dark staining; (b) nuclei then enlarge; (c) the chromatin (contents of nucleus) clumps; (d) and finally the nuclei disintegrate; (e) Only cellular debris is left after the interaction of the cancer cells with BEC; (f) This cell death is characteristic of apoptosis which is also known as programmed cell death.
2.3. The Therapeutic Agents in CuradermBEC5 Kill Cancer Cells in Proliferative and Non-Proliferative Stages Ensuring Rapid Removal of All Cancer Cells
A very important reported aspect of BEC therapy is the killing of cancer cells at their proliferative and nonproliferative stages. These observations are in stark contrast to other well established anti-mitotic chemotherapy drugs that only kill cancer cells while they are dividing and also kill normal cells when they too are dividing [40-48].
Traditional chemotherapy drugs lack specificity, as they enter both cancer and normal cells mainly through diffusion. Due to their DNA reactivity the widely used anticancer drugs can cause a second tumour which may be different than the one originally treated several years after “curative” treatment. BEC ruptures lysosomes and also affects the mitochondria in cancer cells which lead to apoptosis. BEC therapy lacks the mutagenic and carcinogenic potential of currently used chemotherapy drugs [7,29,34,42].
2.4. The Therapeutic Agents in CuradermBEC5 Kill Cancer Cells Only and Not Normal Cells
BEC and its individual components solamargine and solasonine kill cancer cells in a dose-dependent manner and, at therapeutic doses, do not affect normal cells [5,32,34].
Ex Vivo studies have demonstrated that BEC is effecttive against a wide spectrum of human cancers and that BEC is, in a dose dependent manner, selectively killing cancer cells without harming normal cells [11-16,32,34, 47,49-52].
Figure 4 illustrates the specificity of BEC on cancer cells and lack of effect on normal cells. This figure shows BEC at a concentration of 10 µg/mL eliminates various cancer cell lines but has no effect on normal cells such as the sensitive bone marrow cells.
BEC and its individual components solamargine and solasonine have been shown to be very effective in killing other human cancer cells such as Ehrlich carcinoma, K562 leukaemia, colon (HI29) cancer, liver (Hep G2) cancer cells, promyelocytic leukaemia (HL-50) and lung cancer cells but not normal cells [11-20, 50-53].
Table 1 shows at what concentrations solamargine kills cancer cells and normal cells. The safety margin of killing cancer cells relative to normal cells, the Therapeutic Index (TI) is also shown [11-20,50-53]. The higher the TI is, the more effective and safer the anticancer drug is.
MDR refers to multidrug resistant cancer cells which means that these cancer cells are no longer killed by existing anticancer drugs. Solamargine (BEC) does kill these MDR cancer cells! The higher the safety margin, the more effective solamargine is in killing cancer cells relative to normal cells. Safety margins of above 2 are regarded highly beneficial for anticancer drugs.

Figure 4. Effect of BEC on various primary cell lines and cell cultures. This figure shows that at a concentration of 6 ug/ml of BEC, virtually all Ovarian cancer cells are killed but no Bone Marrow cells are affected.
In Vivo single dose studies in mice with the lethal Sarcoma 180 cells have similarly shown that the LD50 and ED50 resulted in a TI of 3.3.
Figure 5 shows the lethal toxicity in normal untreated mice of single i.p. doses of BEC. The LD50 of BEC is 29 mg/kg [1].