1. INTRODUCTION
The passion fruit Passiflora L. are members of the genus (family Passifloraceae), which has a broad genetic base. Some wild species remain wild, others are grown as ornamental plants for food [1,2] and for medical purposes to treat anxiety, insomnia, bronchitis, asthma and hypertension [3]. Most species in Passiflora are native to tropical and subtropical regions of South America, considering Brazil as the center of diversity Pasifloraceae [1,2]. From 400 recognized species of Passiflora, among 50 to 60 are edible. Most of these species are unknown outside their centers of origin and a few species are economically significant, within which include Passiflora edulis f. flavicarpa, P. edulis f. edulis Sims. and P. alata Dryander [4].
The purple passion fruit or gulupa (P. edulis f. edulis), is a perennial climber plant, cultivated worldwide, and used extensively as food. Besides South America countries, the major growing areas are Africa, India, Malaysia, Australia, New Zealand and Hawaii [5]. In Colombia, planted area has been declining in recent years by plant health problems, especially diseases, caused by different groups of pathogens, which include viruses such as Cucumber Mosaic Virus CMV, Soybean Mosaic Virus SMV, Cowpea Aphid-Borne Mosaic Virus CoABMV [6], oil spot caused by Xanthomonas axonopodis [7], vascular wilt root, and collar rot by Fusarium and F. solani, respectively [8], and scab associated with fungi of the genera Cladosporium, Colletotrichum, Alternaria and Botrytis. On fruits, scab causes deterioration in field and postharvest quality damage, attacking also leafs and stems of the plant. On approach to the etiology, diagnosis of scab was based on isolates on agar plates, being different micro-organisms, so it remains without determine the primary causal agent.
Because the scab is a limiting factor in export production of the fruit, it is necessary to determine the causal agent of the disease through a process of diagnosis, including evaluation of the pathogenicity of isolates in the host that allow establish a program of integrated pest management. Based on the above, in this study had aimed to determine the etiologic agent associated with the gulupa scab, its pathogenicity, and describe the infective process of this microorganism in gulupa tissues.
2. MATERIALS AND METHODS
2.1. Isolation of Microorganisms Associated with Scab Symptoms
To obtain microorganisms associated to gulupa plant material with symptoms of scab, was assessed in field crops from municipalities of Venecia, San Bernardo, Granada and Tibacuy (Cundinamarca, Colombia). Leaves, branches and fruits with early and advanced lesions were taken from plants, for be processed on the laboratory of plant pathology at the National University. To isolate of microorganisms associated to symptoms of scab, were superficially sterilized explants with lesions of the disease, planted on potato dextrose agar-PDA(Oxoid®) and incubated for 7 days at 25˚C [9]. Fungal colonies grown on PDA were purified and preserved in 10% sterile glycerol at 4˚C.
2.2. Pathogenicity Tests under Laboratory Condition
monosporic isolates identified as Cladosporium, were grown from 8 to 14 days in SNA (1 g: KH2PO4, 1 g: KNO3, 0.5 g: MgSO4·7H2O, 0.5 g: KCl, 0.2 g: Glucose, 0.2 g: Sucrose and 20 g: Agar for litre) at 25˚C. Then conidia were collected and prepared for each fungus conidial suspension adjusted to a concentration of 1 × 106 conidia/ml. In laboratory, tests were conducted on fruits in state of growth [10], and young leaves detached of healthy plants, which were superficially sterilized [11], later, fruits and leaves were placed on grids confined in plastic containers and inoculated individually with 5 isolates of Cladosporium (FABCl14, FABCl21, FABCl22, FABCl43 and FABCl45) by the following methods of inoculation: T1 tissue wounded + 6 ul: inoculum, T2 inoculum, + 6 ul: tissue without injury, and as controls T3 tissue wounded + 6 ul: sterile distilled water and T4 No wound + 6 ul: sterile distilled water. In each treatment were inoculated 4 fruits and leaves in 3 and 2 points respectively. The organs were incubated in a growth chamber (Thermo Scientific®) at 19˚C/12h. Light and 17˚C/12h of darkness and relative humidity up 95%. The tests were repeated 2 times and the variables evaluated were incubation period and latency on days.
2.3. Pathogenicity Test under Greenhouse Conditions
Under greenhouse conditions, 15 Cladosporium isolates were inoculated individually in gulupa plants on fruits in state of growth [10], and fully expanded leaves, with temperature of 20.2˚C and relative humidity 46.1%. Inoculation methods mentioned above. For each strain were inoculated 3fruits and leaves. Subsequent to inoculation, the organs were covered with plastic bags sprayed inside with sterile destilled water to provide optimum relative humidity (95%) to complete expression of disease symptoms. The variables evaluated were incubation period and latency on days. This test was repeated twice to verify the reproducibility of their results.
With data of latency and incubation periods recorded under greenhouse conditions were conducted analysis of variance in SAS (following a Completely Randomized Design) to establish statistical differences among isolates inoculated on leaves and fruits and were performed tests of Tukey comparison of means to define the most virulent isolates in terms of faster symptom expression and production of inoculum.
2.4. Analysis of Scanning Electron Microscopy (SEM) of Leaves Inoculated with Cladosporium
Leaves of gulupa inoculated with Cladosporium were analyzed by scanning electron microscopy. To this, cuts 1 cm2 of leaf tissue with and without symptoms of scab were fixed in 2.5% glutaraldehyde, dehydrated by consecutive passes of 15 minutes in ethyl alcohol at 25%, 50%, 70%, 90% and 100%, dried critical point for 2 hours and 30 minutes alloy coating gold/palladium carried out in virology laboratory of International Center for Tropical Agriculture (CIAT). Finally, images of sections were analyzed on a scanning electron microscope (Jeol jsm6490Lv) in Scanning Electron Microscopy laboratory of the Andes University.
3. RESULTS AND DISCUSSION
3.1. Isolation of Microorganisms Associated to Scab
In tissues of gulupa with symptoms of scab, were obtained a total of 54 isolates identified to genus level as Cladosporium (38), Colletotrichum (13) and Botrytis (3). Preliminary tests in tissues showed that neither Colletotrichum and Botrytis cause scab lesions in gulupa tissue, and it is happen with Cladosporium [12], it was consistently the most frequently genus isolated, 15 strains of this fungus were selected and evaluated for their pathogenicity in gulupa (Table 1).
3.2. Pathogenicity Tests in Vitro
Obtained results suggest to Cladosporium as the causal agent of scab in gulupa due to the expression of disease symptoms on leaves and fruit after artificial inoculation, consistent with reports of symptoms and etiology of the disease in Brazil and Australia [13]. Conditions to obtain symptom expression were high relative humidity (95%), and a range of temperature of 17˚C - 20.2˚C. Under controlled conditions the fruits inoculated with Cladosporium (FABCl45) exhibited symptoms at 9 and 17 days post-inoculation (DPI) (incubation period), while the expression of signs (generation of new structure of fungus in lession) occurred at 10 and 18 DPI (latency period), a day after appear visual disturbs in tissues (Figures 1 and 2). Symptoms observed on inoculated fruits correspond to the intermediate state of the disease, called a chancre; there was no evolution to the state of showed in field conditions. Fruits with an expression of

Table 1. Origin of isolates of Cladosporium evaluated for pathogenicity in gulupa.

Figure 1. Sequence of symptoms of scab on gulupa wounded fruits inoculated with Cladosporium on in vitro conditions. (A) 3 DPI; (B) 5 DPI; (C) 7 DPI; (D) 9 DPI; (E) 10 DPI and (F) 11 DPI.
symptoms had a progressive collapse of affected tissue, as result of degradation of epidermal and subepidermal cells in the exocarp and sclerotic cells in the mesocarp [14].
In leaves of gulupa were reproduced symptoms of scab disease in 2 of 5 isolates evaluated (FABCl14 and FABCl22). Symptoms of the disease appeared at 8 and 9 DPI by the methods with and without injury, respectively, indicating delays in the incubation period in inoculated leaves by the second method, while the expression of signs occurred at 10 and 11 DPI, two days after the initial symptoms appears. The disease become visible only when is inoculated abaxial surface of leaves, and commenced with the development of small irregular brown spots in adaxial and abaxial surfaces (Figure 3(A)). Subsequently, the spots started to coalesce towards 11 DPI, forming a unique brown spot on which is visible a gray to olive sporulation of the fungus to the 12 - 13 DPI (Figures 3(B) and (C)). Two days later, the injury turns in a deep green color in the periphery and is notorious an advancing leaf senescence, taking place the phenomena of “green islands” (Figures 3(D)-(F)). Although the classic concept of “green islands” has been applied to refer to biotrophic pathogens, this can be induced also by necrotrophic pathogens, and in this case is called “phenotype of green islands” elicited by toxins of fungi, which would be related to production of toxins as has been reported to necrotrophic fungi, including Alternaria brassicae on scabcanola leaves (Brassica napus) [15].

Figure 2. Sequence of symptoms of scab on gulupa not wounded fruits inoculated with Cladosporium on in vitro conditions. (A) 9 DPI; (B) 10 DPI; (C) 11 DPI; (D) Control; (E) PDA re-isolation fruit inoculated with the pathogen, F. Re-isolation of control fruits.

Figure 3. Sequence scab symptoms on leaves of gulupa inoculated with Cladosporium under in vitro conditions. (A) 9 DPI; (B) 11 DPI; (C) 13DPI; (D) 15 DPI; (E) 29DPI; (F) Inoculated leaf showing symptoms of diseases; (G) Re-isolation from inoculated leafs.
The development of symptoms in tissue without injury, proves that Cladosporium pathogenic isolates have the ability to penetrate, invade, reproduced and spread, thus fulfilling the developmental stages of the disease in P. edulis [16], although wounds can also to promote early expression and development of symptoms in leaves. Penetration process may happen after the release of extracellular hydrolytic enzymes such as lipases, pectinases, cellulases and proteases, such as those associated with isolates of C. herbarum recovered in scab lesions in passion fruit in Brazil [17]. Reisolation of inoculated Cladosporium in leaves and fruits allowed the corroboration of the pathogenicity (Figures 2(E) and 3(G)), while in control leaves and fruits inoculated with sterile distilled water there was no expression of symptoms, nor no growth of microorganisms on agar plates as expected (Figure 2(F)), confirming Koch’s postulates.
3.3. Pathogenicity Test in Greenhouse Conditions
Under greenhouse, scab symptoms were reproduced on fruits between 6 and 10 DPI with Cladosporium by the method without injury, and initiated with the formation of spots and brown watery blisters on which there was expression of signs (conidia) of the pathogen between 8 to 12 DPI (Figures 4(A) and (C)). While injuries started to coalesce a result in the intensification of size of them, and among 9 to 13 DPI was an increase in the sporulation of the fungus, as well as the formation of green halo in the peripheral area of the lesion, and secondary infections in other areas of the fruit (Figures 4(D) and (G)); This due primary lesions started to crack in the periphery at 13 DPI, on 15 DPI is observed a hollow cavity in subepidermal zone, as result of the degradation of cells in the mesocarp. In contrast to pathogenicity tests in vitro, in greenhouse conditions, canker lesions evolved Scab, accumulating in the fruit surface layers of cork emerging at 23 DPI (Figures 4(K)-(L)).
Young leaves fully extended develop symptoms among 7 and 11 DPI in tissue wounded and signs of pathogen initiates 1 day after the initial lesions; however in tissue without wounds, incubation periods and latency were delayed 2 to 3 days. In whole plants, the progress of symptoms in leaves showing correspondence with the pathogenicity tests in the laboratory, and initial lesions were circular areas of dark to light brown (necrosis), slightly depressed, on which sporulation of the fungus is visible in the center. In the greenhouse is evidenced by the break of the center of wound (13 DPI), a phenomenon called abscission and occurs in affected leaves under field conditions (Figure 5).
According SEM analysis of tissues inoculated with Cladosporium, leaf topography is altered in the interme-

Figure 4. Sequence of scab symptoms on gulupa fruit inoculated with Cladosporium. (A) 6DPI; (B) 7DPI; (C) 8DPI; (D) 9 DPI; (E) 10 DPI; (F) 11DPI; (G) 12 DPI; (H) 13 DPI; (I) 14 DPI; (J) 15 DPI; (K) 18 DPI and (L) 23 DPI.

Figure 5. Sequence of scab symptoms on gulupa leaves inoculated with Cladosporium under greenhouse conditions. (A) 9 DPI; (B) 10 DPI; (C) 11 DPI; (D) 12 DPI; (E) 13 DPI; (F) 15 DPI; (G) Mature leaf of gulupa plant with scab symptoms induced with and without injury.
diate stages of disease and take place abundant sporulation of the fungus in the middle of lesion, and less extent to periphery (Figures 6-8). Solitary conidiophores arise adjacent to stomata (Figure 9), which differs from tomato leaves infected with C. fulvum, in which the fungus emerges from stomatal cavity in groups with more than 20 conidiophores [18].
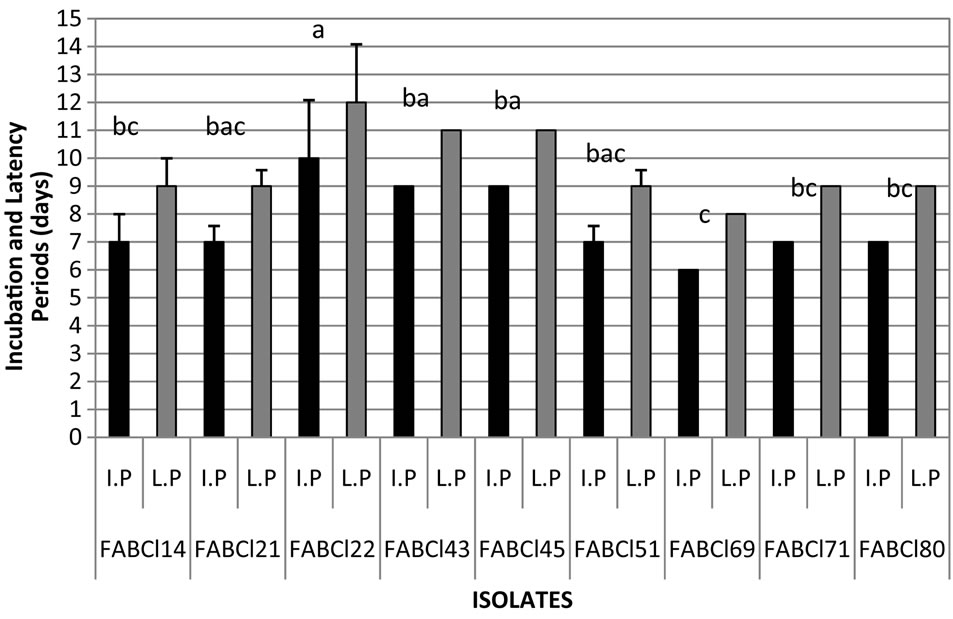
From 15 isolates of Cladosporium evaluated for their pathogenicity in gulupa under greenhouse conditions, 9 induced symptoms of the disease, being pathogenic on leaves and fruits FABCl14, FABCl21, FABCl22, FABC-

Figure 6. Image of a leaf of gulupa by SEM (230×) healthy tissue.

Figure 7. Image of a leaf of gulupa with scab symptoms (SEM 50×).

Figure 8. Ramoconidia of Cladosporium in a leaf of gulupa with scab symptoms (SEM 5000×).
l51 and FABCl80, pathogenic only on fruits FABCl43, FABCl45 FABCl69 and FABCl71 and nonpathogenic to FABCl27, FABCl50,FABCl73, FABCl74, FABCl78, FABCl79. Statistical differences were verified between pathogenic isolates in relation to the incubation and latency periods average, suggesting different levels of

Figure 9. Structures of Cladosporium in a leaf of gulupa with scab symptoms (SEM, 500×).
 (a)
(a)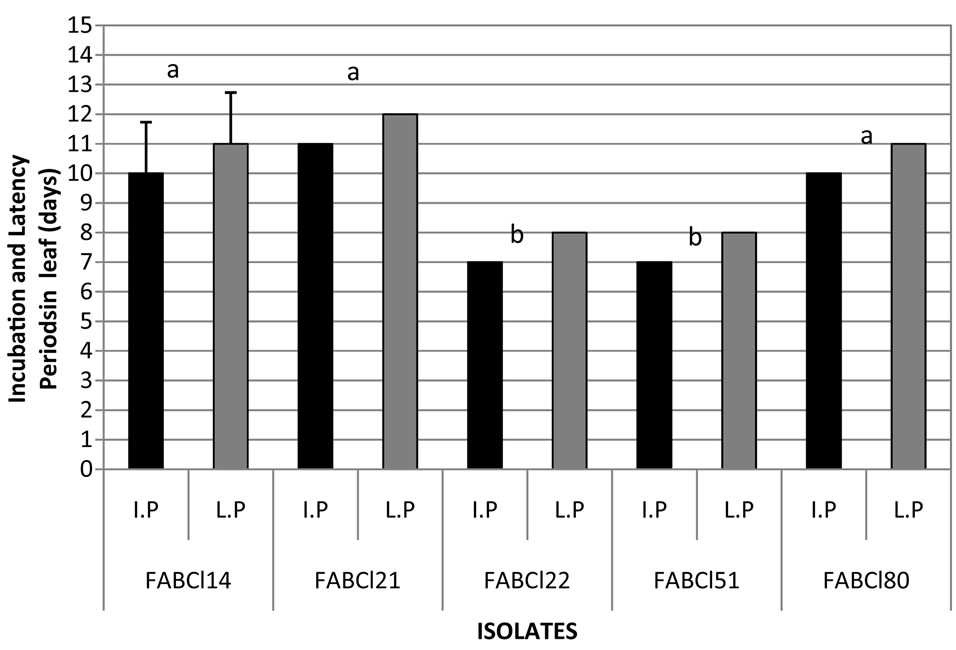 (b)
(b)
Figure 10. Incubation and latency periods of Cladosporium in gulupa recorded in greenhouse conditions. Fruit (a) and leaves (b). *Strains with different letter present statistical differences (P = 0.05).
virulence in the pathogen populations (figure 10). However, is needed additional studies on which can be determinate if the variation of virulence observed in this study is correlated with different species of Cladosporium or the races of the pathogen, as has been documented for C. fulvum in tomato [19-25], so studies are required in that direction, including the development of differential varieties of host plant.
According to the results obtained in this study, can be concluded that Cladosporium is the causal agent of scab in gulupa, because were reproduced symptoms of the disease by artificial inoculation of isolates of the fungus on leaves and fruit under in vitro and greenhouse conditions. Preliminarily, C. lycoperdinum can be the specie involved in this disease, offering some options of control since now is known times of infective cycle of disease [26].
4. ACKNOWLEDGEMENTS
This research was granted by the Ministry of Agriculture of Colombia, Horticultural Association of Colombia—Asohofrucol and Universidad Nacional de Colombia through project“Análisis Epidemiológico y Valoración Económica de las Principales Enfermedades de la Gulupa”. Thanks to Dr. Cristian Olaya—CIAT by technical support.