Dietary Patterns and Empirical Dietary Inflammatory Index in Southern Brazil and Risk of Colorectal Cancer: A Case-Control Study ()
1. Introduction
No communicable diseases, including cancer, are the leading causes of death worldwide, especially in developing countries [1]. Changes in lifestyle signal an increasing impact on the incidence of cancer in the coming decades [2]. The National Cancer Institute [3] estimated that in Brazil, around 625,000 new cases of cancer were registered for the period of 2020-2022, with the most frequent being prostate and CRC in men and breast and CRC in women.
Cancer of the colon and rectum (CRC) has worldwide epidemiological relevance, since it is the third most diagnosed malignant neoplasm and the fourth leading cause of cancer death [2]. Its incidence is increasing rapidly in developing countries, especially among individuals who adopt western-style diets [3] [4]. In Brazil, 20,520 new cases of CRC were estimated in men and 20,470 in women for each year of the 2020-2022 triennium [3].
The risk factors for this condition are well described in the literature, pointing out that CRC is a multifactorial disease influenced by genetic, environmental and lifestyle factors, such as smoking [5] [6], overweight and obesity [7] [8] and eating habits. Among them are remarkable the consumption of alcoholic beverages [9] [10], low intake of fruits, vegetables and cereals [11] and excessive consumption of red meats and processed foods [12], a pro-inflammatory diet which is also known as a Western diet [13]. The dietary inflammatory index [14] [15] has recently been developed to assess the inflammatory potential related to dietary patterns, and this has been used to assess the risk of several diseases, including CRC [16] [17] [18].
Furthermore, a chronic inflammatory process is necessarily accompanied by overgeneration of reactive oxygen species (ROS), which promote deleterious effects to cells and important biological molecules. To prevent or compensate such oxidative challenge aerobic organisms are endowed with several enzymatic and non-enzymatic as well as nutritional antioxidant defenses [19].
Considering that Brazil is a developing country with increasing rates of colon rectal cancer, and that in the state of Paraná, southern Brazil, CRC is the second most frequent neoplasm in women and men, we designed the present case-control study aiming to evaluate whether or not lifestyle and eating habits has correlation with CRC diagnoses in individual living in Southern Brazil. In addition, based on eating habits, we calculate the population’s eDII and correlate it with the risk of CRC, an unprecedented analysis for the Brazilian population.
2. Material and Methods
2.1. Design and Data Collection
The present case-control study was conducted in Cascavel, Paraná, south Brazil (Figure 1), from November/2016 to December/2017. Individuals diagnosed with CRC (cases) and healthy (controls) were evaluated for nutritional status and dietary habits (Figure 2). The cases were patients diagnosed with CRC, confirmed
![]()
Figure 1. Location of the city of study, Cascavel, Paraná, Brazil.
![]()
Figure 2. Study design of individuals with CRC diagnoses and healthy adults living in southern Brazil.
by biopsy and histological analysis, under follow-up at reference institutions in the city area, aged ≥18 years, first neoplasm, in treatment at most for 12 months, lucid and oriented. Patients were recruited while awaiting care or hospitalized. Control subjects were aged ≥18 years, with no diagnosis of intestinal disease confirmed by colonoscopy at most for 12 months. Cases and controls were matched by gender and age (±5 years).
2.2. Anthropometric Assessments
The individuals were submitted to an anthropometric assessment to measure body weight (kg) and height (m) as standardized by the World Health Organization-WHO. For the nutritional diagnosis the Body Mass Index (BMI) was used, considering <18.5 kg/m2 undernourished, 18.5 to 24.9 kg/m2 eutrophic, 25 to 29.9 kg/m2 overweight, 30 to 39.9 kg/m2 as obese and >40 kg/m2 morbid obese [20].
2.3. Dietary Intake Assessment
All subjects were questioned about dietary habits, using a Qualitative and Quantitative Food Frequency Questionnaire [21] validated for CRC patients, composed of 101 items divided into 13 categories.
The interview was conducted by a trained nutritionist and was only recorded after the individual (case or control) signed the informed consent. The patients were questioned about their usual diet before the onset of symptoms or oncological diagnosis, and the controls regarding the usual eating habits. For each food of the questionnaire the individual should indicate the frequency of consumption and the size of the portion ingested in home measures. At the end of the questionnaire the individual could list other foods and beverages that he usually consumed at least once a week.
2.4. Inflammatory Index of the Empirical Diet
Still, from the food frequency questionnaire, the inflammatory index of the empirical diet (eDII) was calculated considering the consumption of 8 pro-inflammatory dietary components (red meat, processed meat, organ meat, other fish, eggs, sugar sweetened beverages, tomatoes and refined grains) and 8 anti-inflammatory components (leafy green vegetables, dark yellow vegetables, fruit juice, oily fish, coffee, tea, wine and beer), scored at −2, −1, 0, 1 and 2 in accord with the frequency of consumption. The final score can vary from −16 to +16, with the highest scores being associated with a pro-inflammatory diet [15].
2.5. Assessment of Other Variables
Information on participant demographic characteristics, medical history of hypertension and type 2 diabetes, family history of cancer and CRC and tobacco use was obtained of standard questionnaire.
2.6. Statistical Analysis
The Student’s t test was used to explore the differences between case and control groups for parametric variables, and comparison of the categorical variables was performed by the chi-squared (χ2) test. For the descriptive analysis of the variables were used means, standard deviations and proportions. The association between diagnosis and explanatory variables was tested using the logistic regression technique. The variable selection method allows the user to specify how independent variables are inserted into the model. In the stepwise selection procedure, statistically insignificant variables are removed from the model from a complete model, where all variables of interest are included. The removal of each independent variable is listed as a separate step and at each step a new template is adjusted. The procedure is interrupted when no more independent variables can be removed from the stepwise model.
In this stage of the work, the decision tree was constructed, using the R (“rpart”) package, for the purely exploratory purpose, in this way there was no distinction between training and test data. The result of the decision tree served as an indicator of which variables are most relevant to cause greater partitioning of the groups in terms of the outcome. In addition, the points of separation of the explanatory variables proposed by the decision tree served as a basis for testing a new categorization of the continuous variables in the logistic regression model. The variables retained formed the final model. The level of significance was set at 5%, with 95% confidence intervals. For data analysis, the R language (3.5.1) was used. The linear regression coefficients were interpreted in terms of odds ratios.
3. Results and Discussion
A total of 356 individual were included in the study, of which 178 patient were diagnosed with CRC (149 cases of colon cancer and 29 cases of rectal cancer) and 178 individuals without intestinal lesion (control), with a prevalence of males (53%) (Table 1). In our study, the male gender had a higher risk of developing CRC (OR 3.34, 95% CI, 1.35 - 8.24, p = 0.007) in relation to the female gender.
A higher prevalence of colon cancer diagnosis compared to the rectum was found in other epidemiological studies, being associated with its extension and longer exposure to dietary carcinogens [8] [22]. In contrast, the lower prevalence of women diagnosed with CRC was also observed in other similar studies [23] [24], attributing the greatest concern with health, in addition to hormonal factors. Some evidence suggests that female hormones, especially estrogen, may be responsible for the protective effect in women [25] [26] because estrogen, when associated with ERβ receptors found in the colonies [27], as well as in biopsies of CRC [28], is capable of inducing apoptosis of tumor cells [29] [30].
In this study, 142 patients (79.8%) were aged 50 years or older, and age was identified as a risk factor for CRC etiology (Table 1). From the age of 60, the risk
![]()
Table 1. Demographic and clinical characteristics of study participants in cases and controls.
increased considerably, and from 60 to 69 years the risk increased by 3.46 times (1.13 - 10.59) and over 70 years increased by 4.07 times (1.09 - 15.25), p = 0.017. Age is characterized as a risk factor for the development of neoplasia, considering some factors such as cellular aging and greater exposure to carcinogenic agents, such as solar radiation and food. In the case of CRC, the WHO recommends that screening for early diagnosis should be intensified after the age of 50 [3]. Our findings corroborate other related investigations [22] [23] [31] where it was possible to verify the prevalence of individuals over 50 years diagnosed with CRC, emphasizing the probable relationship of lifestyle to intestinal carcinogenesis.
The patients with CRC were former smokers was 21.9%, with a higher prevalence among males (Table 1). Ex-smoker was related to the diagnosis of CRC (OR 1.9, 95% CI, 1.18 - 3.06, p = 0.03). Ex-smokers were also associated with higher risk for CRC in our study, corroborating other related findings [5] [6] [31]. Cigarette smoke contains carcinogenic agents such as aromatic heterocyclic amines, nitrosamines and polycyclic aromatic hydrocarbons, which undergo cytochrome P450 metabolism leading to the formation of DNA adducts that can not be repaired, as well as genetic mutation (KRAS, BRAF and MYC) [31].
In relation of cancer family history 104 (58.4%) patients had a history of cancer in family and 45 (25.3%) reported a diagnosis of CRC in parents or grandparents (Table 1). The diagnosis of CRC in the family increased the risk of developing the disease in patients included in this study (OR 1.98, 95% CI, 1.16 - 3.38, p = 0.011). Family history of cancer is also presented as a risk factor for some cancers, including CRC, as found in individual living in Southern Brazil. In the case of CRC, the genetic factor is related to familial adenomatous polyposis (FAP) and Lynch syndrome (LS), with about 1% - 3% of all cases of CRC being especially attributed to LS. The high carcinogenic potential of LS is caused by gene mutations involved with DNA repair, including hMLH1, hMSH2, hMSH6 and PMS2. FAP accounts for approximately 1% of CRC cases and is caused by defects in the APC gene related to tumor suppression [3] [32].
In our study, overweight individuals (BMI > 24.9 and <30 kg/m2) and obese (BMI > 30 kg/m2) (Table 1) presented a higher risk of developing CRC, with OR 3.32 (2.08 - 5.29) and OR 20.85 (6.04 - 72), respectively, with a value of p < 0.001. It was also found that some patients had comorbidities related to being overweight. 73 subjects (41%) used daily medication for hypertension and 28 (15.7%) oral hypoglycemic for control of glycemic levels, as described in Table 1. The diagnosis of type II diabetes increased the risk for the development of CRC (OR 3.97, 95% CI, 1.75 - 8.97, p < 0.001).
Excess weight is also a risk factor in the etiology of CRC. In response to endocrine and metabolic signals, adipose tissue releases innumerable hormones such as leptin, adiponectin and resistin, in addition to cytokines such as TNFα, which promote a state of chronic inflammation and insulin resistance, a condition associated with increased IGF-1 synthesis, the carcinogenic potential for increased cell proliferation [33] [34]. The high prevalence of overweight individuals was associated with an increased risk for CRC, corroborating the findings of an investigation conducted in Arizona-USA [35] and Australia [36], in which patients with CRC were evaluated and overweight was diagnosed in more than 68% of the individuals, with a higher prevalence among males.
Among the comorbidities related to overweight and predisposing to CRC, our study identified a high percentage of patients with type II diabetes (Table 1), corroborating other related findings [37].
Food intake is also closely related to the development of CRC. For this, we initially compared the usual dietary intake of cases and controls. TableS1 and TableS2 show the dietary habits of the main food groups. In this study, the frequency of consumption of coffee (p < 0.001), beans (p < 0.001), vegetables and leaves (p < 0.001), fruits (p < 0.001), processed juice and soda (p = 0.0006), eggs (p < 0.001), red and processed meat (p < 0.001), chicken (p < 0.001) and fish (p < 0.001) was different between individuals with CRC and control group.
The intake of refined or brown bread and rice was also different between CRC and control patients (p < 0.001). Our investigation found that CRC patients had bread and rice intake preferably white (83.7%), while control subjects preferred bread and brown rice intake (57.9%). Ingestion of alcoholic beverages (p = 0.7545) and dairy products (p = 0.08) was no different between groups (TableS1).
The CRC patients reported fish consumption less than once a week (46.1%) (p < 0.001). Chicken intake at a frequency of 3 to 5 times per week was higher in CRC patient group (60.7%) (p < 0.001), as described in TableS2; however, chicken and fish intake was not associated with development CRC (Table2).
Meat is an important dietary source of protein and iron and is routinely consumed in Western countries such as Brazil, especially in the southern states [12]. Our investigation found that patients with CRC and the control group had a different pattern regarding the intake of red and processed meat, and in control group it was found that 89 (50%) individuals consumed red meat more than three times a week while in the CRC group we found 173 (97.2%) of the patients. Regarding the intake of processed meat, 161 (90.4%) patients in the control group reported consumption up to twice a week, whereas in control group most individuals (63%) had consumption ≥ 3 times/week (TableS2). In addition to
![]()
Table 2. Colon rectal cancer risk factors of participants according to dietary patterns. OR Odds ratio. The OR was adjusted for egg, chicken and fish intake (servings/week) and dairy, coffee, vegetables, leaves, industrialized juice, soda and alcoholic beverage intake (servings/day).
the frequency of consumption, the amount consumed was evaluated, and again there was a higher intake in the CRC group, as described in Figure 3(a).
Patients with CRC presented higher intake of red meat (113.72 g/day), processed meat (26.76 g/day), chicken and fish (100.17 g/day) compared to the control group (90.26; 4.94 and 49.15 g/day), respectively (p < 0.05) (Figure 3(a)).
The weekly intake (g) of red and processed meat was higher in the CRC group (983.48) compared to the control group (666.53) (p < 0.001), being even higher among CRC men (1097.25) compared to CRC women (853.25) (p < 0.001), nevertheless, in this study, red meat was not associated with CRC (Figure 3(b)).
In several epidemiological studies the high consumption of red and processed meat was associated with the risk of CRC [38], other studies strongly associated the consumption of red meat prepared at high temperatures with the risk of CRC [39]. However, in our study, red meat intake was not associated with intestinal carcinogenesis, as found in other investigations [40] [41].
In our research we also found the intake of chicken and fish as alternatives for the replacement of red or processed meat; however they were not related to a protective effect on colorectal carcinogenesis. In contrast, in other related studies increased fish consumption and concomitant reduction in red meat intake promoted lower formation of nitrous compounds (NOCs), which have carcinogenic
![]()
Figure 3. (a) Red meat, processed meat, chicken and fish intake (g/day) among CRC and control patients; (b) Vegetables, leaves and fruits intake (g/day) among CRC and control patients; (c) Red and processed meat intake (g/week) and fruits, vegetables and leaves (g/day) among CRC and control patients. Results were expressed in quartiles. The lower horizontal bar outside the box has the lowest identified value (excluding outliers), the horizontal bar at the bottom of the box represents the first quartile (P25), the horizontal bar inside the median box, the horizontal bar at the top of the box the third quartile (P75) and the upper horizontal bar outside the box the highest identified value (excluding outliers). The circles represent the outliers. (***) denotes statistically significant difference p < 0.001 compared to the control group.
potential, suggesting a beneficial effect on the risk for CRC [42]. Finally, in a study conducted in Brazil [24] patients with CRC who reported a higher consumption of beef, chicken and pork compared to controls, correlated with an increased risk for carcinogenesis in 1.025, 1.069 and 1.121, respectively.
In order to prevent intestinal carcinogenesis, the World Cancer Research Fund-WCRF recommends a weekly consumption of up to 500 g of red meat and minimal or sporadic consumption of processed meat in order to reduce the development of CRC [43]. The strongest association of colorectal carcinogenesis and ingestion of processed meats also seems to be related to the additives used in the meat industrialization process, since in addition to salt, nitrites and nitrates are added and these are considered human carcinogens according to WCRF because they can be converted to nitrous compounds [44]. In addition, the presence of heme iron also contributes to intestinal carcinogenesis by the mechanisms described above [45].
In our study, consumption of processed meat was associated with increased CRC. These results corroborate the result observed after following a cohort for seven years, where there was an increased risk of colorectal carcinogenesis related to the intake of processed meat (HR 1.16; 95% CI 1.01 - 1.32; p = 0.017), with potential mechanisms related to heme iron (HR 1.13; 95% CI 0.99 - 1.29; p = 0.022), presence of nitrate (HR 1.16; 95% CI 1.02 - 1.32; p = 0.001) and production of heterocyclic amines (HR 1.19; 95% CI 1.05 - 1.34; p < 0.001) [46]. Similar results were reported in a case-control study conducted in Jordanian hospitals suggesting that consumption of red and processed meat may be associated with the risk of developing CRC [47].
In addition, it was observed that 66 (37.1%) CRC patients had lower intake than the recommendation dairy of beans, compared to control subjects (12.3%) (p < 0.0001). Only 63 (35.4%) patients with CRC had ingested at least 3 daily portions of leafy vegetables, while the control group 72.4% of subjects reported adequate intake. Regarding fruit intake, the same behavior was observed, that is, 25 (14.1%) and 128 (71.9%) patients of CRC and control group, respectively, had ingested more than 3 servings daily (TableS1). Vegetables (137.70 g/day) and fruits (258.77 g/day) intake was lower in the patients with CCR compared to control group (195.00 and 372.24 g/day), respectively (p < 0.005) (Figure 3(c)). The daily intake (g) of vegetables, leaves and fruits it was found that CRC patients group had a lower daily intake than the control group, being 395.93 and 564.06 g, respectively, with significant difference between groups (p < 0.001). In the analysis by gender, there was a difference in consumption between men with CRC and control, being respectively 386.99 and 580.57 g/day (p < 0.001). The same behavior was observed in women, with daily intake from the CRC group 406.16 g/day and from control group 545.17 g/day (p < 0.001). There was no difference between intake of men and women with CRC (Figure 3(b)).
The consumption of white bread (Adj. OR 1365.8; 95% CI 3.659 - 10.78; p < 0.001) and processed meat up to two and five times a week (Adj. OR 227.355; 95% CI 2.084 - 8.769; p < 0.001) increased the risk for CRC, in addition to eating up to two servings of fruits daily (Adj. OR 40.57; 95% CI 0.183 - 7.23; p = 0.039). Intake of whole grain bread (Adj. OR 0.04; 95% CI −6.207 - 0.236; p = 0.034) was associated with reduced risk of CRC and red meat consumption was unrelated to the outcome analyzed (Table 2).
Foods such as beans, lentils and chickpeas, classified as oil seeds, are also important dietary sources of protein, however, less associated with the development of CRC, possibly because they are sources of non-heme iron, fiber and minerals, as well as their high content of α-tocoferol, a very powerful nutritional antioxidant cancer [19]. The recommended intake is one serving daily; however, in this investigation, it was observed that patients with CRC consumed beans less frequently than recommended.
Daily consumption of a balanced diet including beans, vegetables, leaves and fruits can minimize the deleterious effect on health by consumption of dietary carcinogens, since these foods contain several antioxidants, which may be sufficient to control the overgeneration of reactive oxygen species before they cause oxidative damage to important biomolecules and cell membranes [13] [19]. Some micronutrients with antioxidant and anti-carcinogenic potential described in the literature include vitamins A, E, C and zinc [48] [49] [50] [51] [52], as well as a panoply of polyphenols.
Our findings are similar to other related studies in which CRC patients, compared with controls, rarely consumed fruits and vegetables (OR 20.8), and a tendency to consume red meat 2 - 3 times a week (OR 3,8) or more than four times a week (OR 9.4) [8], emphasizing the relationship between dietary pattern and colorectal carcinogenesis. Fresh fruit consumption was also inversely correlated with CRC incidence (p = 0.012) in a study [22].
In addition to the frequency of consumption there was a daily intake (grams) of vegetables, leaves and fruits. As showed in Figure 3(b) patients with CRC consumed less daily intake of these foods compared to the control group. To prevent CRC, WHO recommends a daily intake of at least 400 grams of non-starchy fruits and vegetables, emphasizing the risk associated with insufficient intake, as found in our research and corroborated by other similar studies [8] [53].
In addition to the intake of micronutrients, the consumption of dietary fiber may contribute to the reduction of the colorectal carcinogenesis process, considering that the decrease in intestinal transit time implies a lower permanence of the dietary carcinogens in the lumen. The protective effect of the fibers is also related to the increase of the fecal volume, promoting the dilution of toxic compounds in the lumen, fermentation by the colonic flora producing short chain fatty acids, among them butyrate, related to several effects, energy supply to the colonocytes, reduction of intestinal permeability and inflammation of the mucous, reduction of cellular proliferation and increase of apoptosis [54].
In a related study [55] with patients diagnosed with CRC no statistically significant association for the protective effect of fibers on colorectal carcinogenesis (OR 0.85; 95% CI 0.7 - 1.03) was found. Nevertheless, by separating the anatomical region from the disease, the ingestion of total fibers reduced the risk of distal colon cancer (HR 0.62; 95% CI 0.41 - 0.94). In our investigation, the consumption of bread and white rice was associated with increased risk for intestinal carcinogenesis. In the other hand, the consumption of bread and brown rice had a protective effect, in agreement with other related epidemiological studies [8] [56].
Finally, we found that eDII had high scores for men and women with CRC, with an average of 6.07 ± 1.41 and 4.41 ± 1.41 respectively. The stratification of scores in quartiles allowed us to verify that the individuals in the control group, regardless of gender, were concentrated in the lower eDII scores (93.6% males and 80.7% females), while individuals with CRCs were mostly in quartiles 3 and 4, with statistical difference between cases and controls (Table 3).
The risk analysis showed that eDII greater than 3 and 0 increased the risk for CRC in men (OR 66.75; 95% CI 19.05 - 233.93; p < 0.001) and women (OR 55.83; 95% CI 6.53 - 477.16; p < 0.001), respectively. In addition, eDII scores above 5 for males and 2 for females were even more associated with increased risk (Table 3).
The empirical dietary inflammatory index calculated in this study corroborates the results presented above, showing that the individuals in the control group have scores that characterize a diet with an anti-inflammatory profile, unlike patients with CRC. In our investigation, high eDII scores (>3 for men and >0 for women) were associated with an increased risk of CRC (OR 66.75 and 55.83, respectively). The inflammatory potential of the diet has already been associated with an increased risk of CRC in other investigations [57] [58] [59]
![]()
Table 3. Empirical dietary inflammatory index (eDII) and risk of colon rectal cancer.
emphasizing the importance of using this index to assess the dietary pattern of a population. As far as we are aware, no previous publication has assessed the dietary inflammatory index associated with CRC risk in Brazil.
A systemic and prolonged oxidative stress such as promoted by the Western diet, which is consistently associated with a chronic inflammation process and a concomitant low nutritional antioxidant intake, is apparently associated with an increased risk for carcinogenesis, including CRC. On the other hand, the panoply of nutritional antioxidants that characterizes diets involving grains, seed oils, vegetables and fruits, such as the so-called Mediterranean diet [13], are probably reflecting the benefit of such diets to prevent CRC as well as other types of cancer when compared the so-called Western diet.
4. Conclusions
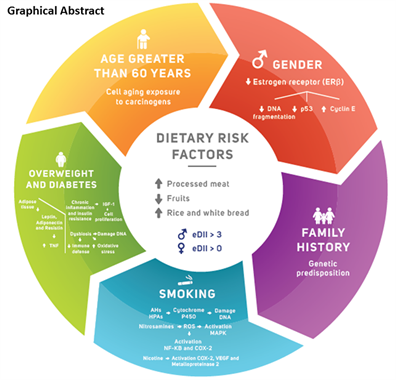
The Western dietary pattern was characterized by high intake of red and processed meat and reduced dietary fiber intake (grains, cereals, fruits and vegetables), which favors were associated with the diagnosis of CRC in the investigated southern Brazil population, as previously verified in other investigations. In an unprecedented way for the Brazilian population, we associate eDII with the risk of CRC. In addition, male gender, smoking and overweight were also associated with increased risk for intestinal carcinogenesis. The graphic summary presents the variables related to colorectal carcinogenesis in our population.
Some methodological limitations of this study need to be considered. First, as in other case-control studies that investigate prior feeding in diagnosis, this research may suffer from memory bias. To try to minimize this effect, the patients included in this study had been diagnosed with CRC at most 12 months. Second, in investigating the relationship between smoking and CRC, the amount of cigarettes consumed daily and the time of use/exposure were not investigated, since the questionnaire used was limited to individuals separated into current smokers, ex-smokers and never smokers. Finally, when investigating the individuals’ usual diet, no information was requested regarding the use of nutritional supplements, multivitamins and/or hormone replacement therapy.
Funding
This work was supported by CNPq and CAPES do Brasil. R.C.P (Proc. 302404/2017-2) receives research grants from CNPq, R.G.E.D. and D.C.S. are CAPES/CNPq scholarship holders.
Supplementary
![]()
![]()
Table S1. Dietary habits for the main food groups between case and control participants. p-values were obtained by Chi-square test (χ2).
![]()
![]()
Table S2. Weekly meat and egg consumption between cases and controls. p-values are obtained by Chi-square test (χ2).