Effect of Sodium Oleate Concentration Variations on Froth Flotation of Manganese Ore ()
1. Introduction
Metallurgical grade manganese ores are located in only four countries: Gabon, South Africa, Australia and Brazil. Hence, the low-grade deposits located in many countries including Nigeria need to be beneficiated to metallurgical grade before utilization in iron and steel companies [1] . Each type of these manganese deposits is a problem by itself in the matter of selection of a proper method of concentration, depending on the manganese minerals and their gangue constituents. Beneficiation technology as applied to manganese ores is similar to that of iron ores. Most ores are crushed and screened, with the lump product (+6 mm) generally being smelted and the fine product (–6 mm) used as feed for chemical and/or electrolytic processing. Sink-float, jigging, tabling, flotation and high-intensity magnetic separation are usually used to upgrade fine manganese ore. Physical separation technologies such as flotation and roasting, and chemical separation process such as leaching have been developed for beneficiation of lower grade and more refractory resources [2] .
The possibility of upgrading low-grade Manganese ore using gravity and magnetic separation technique was investigated by [3] . Shaking table gave a concentrate of 48.89% Mn, spiral concentrator gave a concentrate of 49.34% Mn while magnetic separation gave a concentrate of 52.63% Mn. The corresponding recoveries of the concentration methods were 97.60%, 99.10% and 77.78% respectively, from an initial grade of 38.67% Mn. The results obtained from the tests showed that magnetic separation gave a better recovery of the concentrate compared to the gravity concentration techniques.
[4] Performed microflotation tests with manganese minerals (rhodonite and rhodochrosite) and quartz mineral, using sodium oleate as the collector. The influence of water glass (sodium silicate) as a depressant in the recovery of these minerals with sodium oleate was also investigated. Potential zeta determinations were carried out with the three minerals conditioned with deionised water and with the reagents. It was found that, at a pH of 9, the recoveries of rhodonite and quartz were maximised, and for the rhodocrhosite mineral, the maximum recovery was achieved at pH 11. The water glass at pH 9 was more efficient in depressing quartz than rhodonite, especially for smaller concentrations, whereas the flotation response of rhodochrosite was only slightly affected at both pH 9 and pH 11.
Development of linoeate hydroxamic acid to float a low-grade manganese ore was carried out by [5] . The performance of this LHA and oleic acid (OA) used for anionic froth flotation was compared, and the critical factors of rhodochrosite flotation were investigated. It demonstrated that the use of sodium carbonate instead of sodium hydroxide as the pH regulator, dosages of depressant and collector, and the addition of synergists are essential to the effective recovery of Mn in the flotation.
The separation of mineral by froth flotation technique depends primarily on thedifference in the wettability of particles. Particles to be floated must selectively attach to the air bubbles, so they must be hydrophobic [7] . A few minerals like sulfur, are naturally hydrophobic. Meaning that they can be floated directly, but most minerals are hydrophilic and had to be made hydrophobic by selected surface-active chemicals called collectors. These chemicals selectively coat or adsorb on the desired minerals, usually assisted by any of a number of auxiliary reagents. Most of the auxiliary reagents assist flotation by adsorbing selectively on the particles or by complexing with the chemical species [8] . However, this work investigated the flotation of this ore at 180 µm liberation size of madaka manganese ore [9] .
True flotation utilizes the differences in physicochemical surface properties of particles of various minerals. After treatment with reagents, such differences in surface properties between the minerals within the flotation pulp become apparent and, for flotation to take place, an air bubble must be able to attach itself to a particle, and lift it to the water surface. Figure 1 illustrates the principles of flotation in a mechanical flotation cell. The agitator provides enough turbulence in the pulp phase to promote collision of particles and bubbles which results in the attachment of valuable particles to bubbles and their transport into the froth phase for recovery.
2. Materials and Method
2.1. Materials
Manganese ore samples were collected from two pits at the mine site in Madaka village, Rafi Local Government Area of Niger State. The co-ordinates of the collection pits were: Pit A: N 10˚03'03.0", E 06˚27'50.5"; Pit B: N: 10˚03'03.0", E: 06˚27'55.5". The manganese ore samples collected weighed 30 kg and were securely preserved in a bag.
2.2. Methods
2.2.1. Chemical Analysis of the Crude Ore Sample
Chemical characterization of the crude ore sample was carried out using Energy Dispersive X-ray Fluorescence Spectrometer, model: Shimadzu EDX-702HS operated at 40 kV and 18 mA. The current was automatically adjusted (maximum of 1 mA). A 10 mm collimator was chosen. The counting time was 100 seconds for all measurements. The intensity of element Kα counts per second (cps/µA) was obtained from the sample X-ray spectrum using the EDX Shimadzu software package.
2.2.2. Mineralogical Analysis of the Crude Ore Sample
The Mineral phases in the ore were determined using X-Ray Diffraction (XRD). Ore sample was placed in a lucite holder on the goinometer of the Shimadzu XDS 2400H diffractometer. It was also configured with a graphite monochromator. The diffraction beam monochromator operated at 40 KVA and a current of 30 mA with the 2θ values varying from 0˚ to 80˚ with step size of 0.02˚ for 120
![]()
Figure 1. Contact angle between bubble and particle in an aqueous medium (Source: [6] ).
minutes to create x-ray patterns with enough intensity to produce lines to identify minerals at the 2θ angles. Scanning rate was 0.75 degree per minute. Minerals were identified using the JCPDFWIN software of the Joint Committee on Powder Diffraction Standard (JCPDS).
Scanning electron microscopic analysis (SEM) with EDS of the sample was also carried out using TESCAN SEM and X-Max Oxford EDS with VEGA TC software operated at 20 kV irradiation.
2.2.3. Froth Flotation Concenration
Sample was ground to 180 µm liberation size, washed and subjected to froth flotation experiment using Denver D12 flotation machine. The froth flotation cell was set, charging it with 4:1 water to sample dilution ratio, following the standard operating procedure of Denver flotation machine. 200 g of sample was added to 800 ml of distilled water. The froth flotation was carried out at varied collector (sodium oleate) concentrations (3 g/kg, 7 g/kg and 10 g/kg). Other chemical reagents added were sodium silicate (depressant) and fuel oil (frother), at constant concentrations of 1 g/kg and 4 g/kg respectively Table 1. The Pulp pH was maintained at 9 and was conditioned for five minutes, aerated and floated for fifteen minutes at 1400 rpm impeller speed. The float fraction was scooped and weighed. Both the float (concentrate) and sink (tailing) fractions were taken for chemical analyses.
3. Results and Discussion
The results of all experiment carried out were presented in Tables 2-4 and Figure 2.
3.1. Physical Examination of the Crude Ore Sample
The hand specimen examined physically shows grayish-black with some patches of white and non-magnetic substance with hardness number greater than 5.5 on Mohr hardness scale. The ore was characterized for its elemental content, mineral phases, and morphology and particle size.
![]()
Table 1. Reagent Combination for the flotation experiment.
![]()
Table 2. Chemical compositional analysis of Madaka manganese ore as obtained from XRF.
![]()
Figure 2. XRD image of crude sample of Madaka manganese ore.
![]()
Table 3. Result of chemical analyses of concentrate and tailing at varied sodium oleate concentrations.
![]()
Table 4. Metallurgical accounting of the beneficiated ore at varied collector concentrations.
3.2. Chemical Compositional Analysis of Crude Madaka Manganese Ore
The percentages of chemical compositions of the crude manganese ore sample are shown in Table 2. It could be seen that MnO having 48.44% is the highest chemical compound present, which in agreement with the findings of [4] who put the percentage composition of the raw Madaka manganese ore at 49.5% MnO. This confirms the existence of manganese in the ore sample. Other chemical compounds present in the ore are: Fe2O3 (12.32%), SiO2 (3.1%), Al2O3 (2.37%), TiO2 (0.64%), CaO (0.17%), Cr2O3 (0.07%), Na2O (0.04%), K2O (1.86 %) and SO3 (0.08 %). This grade of manganese is called blast furnace grade according to [10] .
3.3. Mineralogical Analysis of the Crude Madaka Manganese Ore Sample
Figure 2 shows the mineral phases present in the crude sample of the ore. Peaks were identified as that of magnosite (MnO), spessartine (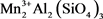 ), Hausmannite (
), Braunite (
) jacobsites (MnFe2O4), Tephroite (Mn2SiO4). The gangue minerals are Vermuculite (Mg, Fe2+, Fe3+)3[(Al, Si)4O10](OH)2·4H2O), Annite (
), and Clinochlore ((Mg, Fe2+)5Al(Si3Al)O10(OH)8), Quartz (SiO2). The ore XRD analysis shows that ore occurs in the form of oxides and silicates.
), Hausmannite (
), Braunite (
) jacobsites (MnFe2O4), Tephroite (Mn2SiO4). The gangue minerals are Vermuculite (Mg, Fe2+, Fe3+)3[(Al, Si)4O10](OH)2·4H2O), Annite (
), and Clinochlore ((Mg, Fe2+)5Al(Si3Al)O10(OH)8), Quartz (SiO2). The ore XRD analysis shows that ore occurs in the form of oxides and silicates.
3.4. Beneficiation of Madaka Manganese Ore Using Froth Flotation Process
The float (concentrate) and the sink (tailing) fractions obtained from this process were characterized and the result presented in Table 2, which shows that, at sodium oleate concentration of 3 g/kg, the grade of manganese was 65.2%, while the grades of other constituents were: Si (4.58%), Al (4.32%), Fe (9.39%), Ti (0.08%), Ca (0.700%), Pb (0.01%), Mg (0.13%), Na (0.06%), K (0.32%). At sodium oleate concentration of 7 g/kg, manganese grade was 62.03%. others were: Si (3.76%), Al (3.40%), Fe (9.48%), Ti (0.67%), Ca (0.75%), Pb (ND), Mg (0.18%), Cr (0.02%), Na (0.05%), K (0.30%), S (0.03%). When sodium oleate concentration was increased to 10 g/kg the Mn grade was 61.30, while the grade of other constituents were: Si (4.52%), Al (3.93%), Fe (9.67%), Ti (0.10%), Ca (0.81%), Pb (ND), Mg (0.16%), Cr (0.02%), Na (0.04%) and K (0.29%). The highest grade of 65.20% of manganese was obtained at collector concentration of 3 g/kg. This shows an inverse proportional relationship to the amount of concentrate recovered.
3.5. Metallurgical Accounting for the Froth Flotation Process
Metallurgical accounting is used to deduce operational mass balance in order to assess the plant performance and control operation.
Table 3 shows the values of percentage weight of feed (F), % grade of feed (f), % weight of concentrate (C), % grade of Mn in concentrate (c), % grade of tailing (t), % metal recovery, concentration ratio and enrichment ratio obtained at different concentrations of sodium oleate. The values of percentage metal recovery, concentration ratio and enrichment ratio can be obtained using Equations (1) (2) and (3) respectively. The respective values of feed (F) and grade of feed (f) remain constant at 100% and 37.5% respectively throughout the experiment. The result in Table 3 reveals that, at 3 g/kg, 7 g/kg and 10 g/kg sodium oleate concentrations, manganese recoveries were respectively 8.87%, 14.30% and 19.61%, while the grades were 65.20%, 62.03% and 61.3% respectively. The result thus showed that the higher the collector concentration, the higher the recovery, but the lower the grade of the metal recovered into the concentrate. These results revealed that 10 g/kg sodium oleate concentration produced the highest recovery of 19.61% but the lowest grade of 61.30% manganese while 3 g/kg yielded the least recovery of 8.87% with 65.29% grade. This showed an inverse proportional relationship between recovery and grade of metal in the froth flotation process which is in agreement with the previous finding of [11] .
Likewise, Table 3 revealed the concentration ratio and the enrichment ratio for all the collector concentrations. The concentration ratios (C/F) are 18.70, 11.6 and 8.33 at collector concentrations 3 g/kg, 7 g/kg and 10 g/kg respectively, this implies that at sodium oleate concentrations of 3 g/kg, 7 g/kg and 10 g/kg 1 ton of manganese mineral concentrate would be produced from every 18.70 tons, 11.56 tons and 8.33 tons of feed respectively. Furthermore, Table 4 shows an inverse proportional relationship with the percentage metal recovery. In addition, another important metallurgical accounting parameter that can be found in Table 4 is the enrichment ratio which is the ratio of the grade of concentrate to the grade of the feed (c/f). The result obtained revealed that the enrichment ratio at collector concentrations of 3 g/kg, 7 g/kg and 10 g/kg are 1.74, 1.65 and 1.63 respectively. Hence, 3 g/kg collector concentration produced the highest enrichment ratio of 1.74, which indicates that at sodium oleate concentration of 3 g/kg, the grade of the manganese in the concentrate is 1.74 times higher than 1% manganese grade in the feed. Furthermore, enrichment ratio equally shows an inverse proportional relationship with recovery.
Parameters such as recoveries, enrichment ratio and concentration ratio are determined using the following formula:
(1)
(2)
(3)
where F, C and T are the weights of Feed, Concentrate and Tailing respectively while f, c and t are their respective assays. The result is shown in Table 4.
While each of these single calculated values is useful for comparing flotation performance for different conditions, it is most useful to consider both the grade and the recovery simultaneously, using a “Grade/Recovery Curve”. This is a graph of the recovery of the valuable metal achieved versus the product grade at that recovery.
4. Conclusions
1) The chemical composition of the ore revealed the following elements in their compounds: 3.10% SiO2, 2.37% Al2O3, 12.32% Fe2O3, 0.64% TiO2, 0.17% CaO, 48.44% MnO, 0.07% Cr2O3, 0.04% Na2O, 1.86% K2O, 0.08% SO3.
2) The mineralogical characterization of the ore shows that the ore consists of the following minerals: Magnosite (MnO), Braunite (Mn2+Mn3+6[O8SiO4], Hausmannite
, Spessartine [Mn2+3Al2(SiO4)3], Manganese dioxide (MnO2), Annite
, Manganosite [MnO], Quartz [SiO2] and Manganese Silicate [Mn2+SiO2] as the major minerals.
3) There exists an inverse proportional relationship between recovery and the mineral grade while enrichment ratio and concentration ratio share a direct proportional relationship with a mineral grade in a froth flotation process.