Efficacy of a Single Oral Administration of Afoxolaner Alone or in Combination with Milbemycin Oxime against Ixodes hexagonus Ticks in Dogs ()
1. Introduction
Ticks are quite common ectoparasites, with over 800 species described worldwide, and more than twelve different species are recognized as parasites of dogs in Europe. The clinical significance of tick infestation in dogs is not only based on direct pathogenic effects (e.g. skin reactions and anemia in case of heavy burdens of ticks), but mainly linked to them as potential vectors. Transmitted pathogens include viral, bacterial, and protozoan agents, among which some may result in severe or life-threatening diseases [1] and/or are zoonotic.
Widespread and endemic in Europe, Ixodes species are known vectors of several pathogens of animal and human significance (e.g. Borrelia burgdorferi, Anaplasma phagocytophilum, Tick-Borne Encephalitis virus…).
The most common tick species parasitizing dogs in Western, Central and Eastern Europe is Ixodes ricinus, also called the wood tick. Other Ixodes species such as Ixodes hexagonus, and Ixodes canisuga, may be found in dogs but their prevalence is lower [2] - [9] .
The so-called Hedgehog tick, Ixodes hexagonus, is a nidicolous tick which has been described in many parts of Europe such as UK and Ireland [3] [8], France [4] [10], Belgium [9], Netherlands [6], Germany [11], Iceland [12], Poland [13], Switzerland [14], Italy [15], and Spain [16] . While less prevalent than Ixodes ricinus, I. hexagonus seems to be the second most common tick found on dogs in some parts of Western Europe. It has been reported to represent 1.2% to 5.6% of ticks collected on dogs examined in recent studies [14] [15] . Other studies have shown that amongst tick-infested dogs, 8.8% to 22.6% of them carry I. hexagonus [8] [9] [11], even if in low numbers.
While Ixodes hexagonus is mainly an endophilic tick species, typically living in its host’s nest, it is able to infest and feed not only on medium-sized wild carnivores such as foxes and mustelids, but also on domestic mammals—cats or dogs, and may attach to humans. It usually does not feed on birds. The hedgehog tick may be observed in urban and suburban areas, with urban parks and gardens potentially supporting significant populations of this tick species [3] .
Ixodes hexagonus is a proven vector of the spirochete Borrelia burgdorferi, the causative agent of Lyme disease [17] [18], and is suspected to be the vector of Theileria annae (syn. Babesia microti-like—Babesia vulpes [19] ) in dogs in Spain [16] . It has also been found to harbour Rickettsia helvetica, Anaplasma phagocytophilum, the Tick-Borne Encephalitis (TBE) virus, and its capacity as a vector has been suggested for all of these pathogens [6] [20] [21] .
Previous experimental and field studies have demonstrated that afoxolaner is highly effective against existing and new infestations of several tick species in dogs for at least 4 weeks [22] - [29], but its efficacy against Ixodes hexagonus ticks has not been examined.
The objective of the present laboratory study was to evaluate the efficacy of afoxolaner in both marketed formulations (NexGardÒ-afoxolaner and NexGard SpectraÒ-afoxolaner plus milbemycin oxime-chewable tablets), administered once orally at the minimum recommended dose against induced Ixodes hexagonus infestations for a period of four weeks.
2. Materials and Methods
The general study design followed the Committee For Medicinal Products for Veterinary Use (CVMP), “Guideline for the testing and evaluation of the efficacy of antiparasitic substances for the treatment and prevention of tick and flea infestation in dogs and cats”, EMEA/CVMP/EWP/005/2000-Rev.3, as well as the World Association for the Advancement of Veterinary Parasitology (WAAVP) second edition guidelines for evaluating the efficacy of parasiticides for the treatment, prevention and control of flea and tick infestation on dogs and cats [30] . The EMA guideline recommends the sample size to get statistically significant results (at least 6 animals per treatment/control group), as well as the schedule of operations regarding the time points for treatment and infestations to assess both curative and persistent efficacy.
The study was blinded—staff members involved in data collection and analysis or health observations had no information about the dogs’ group allocation— and was conducted in accordance with the Good Clinical Practice Guideline (VICH GL9).
2.1. Animals
The sample size was defined according to guideline recommendations in order to get 3 groups of 8 dogs: twenty-four purpose-bred Beagle dogs, 12 females and 12 males, aged 8 to 12 months and weighing 9.1 to 14.5 kg were enrolled.
Acclimation of the animals to the study conditions began 8 days prior to treatment (Day-8). Each dog underwent a physical examination by a veterinarian, on the first day of acclimation, and all dogs were confirmed in good health. They were fed a standard commercially available animal food. Water was available ad libitum.
No animal had been treated with any ectoparasiticide compound for three months before acclimation started.
2.2. Allocation to Treatment Groups
The study followed a randomized block design based on pre-treatment counts of live attached female ticks. A pre-treatment infestation and count was used to form blocks. The twenty four dogs were infested on Day-7 and tick counts were performed on Day-5. Dogs were ranked by decreasing live attached female tick count (dogs with the same tick count were ranked by decreasing identification numbers), and eight blocks of three dogs each were formed. The three dogs with the highest tick count formed Block 1; the next three highest tick counts formed Block 2, and so on, until all dogs were allocated.
Within blocks, dogs were then randomly allocated to one of the three treatment groups, using the PLAN procedure of SAS (version 9.4): Group 1, untreated control, Group 2, NexGardÒ (afoxolaner), and Group 3, NexGard SpectraÒ (afoxolaner + milbemycin oxime).
Twenty four dogs were initially selected for the study, but one female dog from the NexGard Spectra treated group was excluded on Day-1 due to potential pregnancy.
Animals were group-housed in cages by treatment group and by sex throughout the animal phase except on days between tick infestations and counts where the animals were kept individually.
2.3. Infestations, Treatment, and Tick Counts
Treatments were administered orally, once, on Day 0. Starting two days before treatment (Day-2) all dogs were infested with 50 unfed adult (40 females + 10 males) Ixodes hexagonus ticks, and new infestations were conducted on Days 7 and 28. Tick counts were performed at ∼48 hours post-treatment (Day 2) and at ~48 hours following each post-treatment infestation (Days 9 and 30). For each tick infestation, the dogs were sedated for at least 30 minutes, using intramuscular (IM) or intravenous (IV) injection of medetomidine and ketamine, to allow ticks to crawl and select an attachment site. Sedations were reversed using IM injection of atipamezole.
Specific pathogen free Ixodes hexagonus ticks were provided by Utrecht Centre for Tick-borne Diseases (Faculty of Veterinary Medicine, the Netherlands) and by Biology Centre ASCR (Ceske Budejovice, Czech Republic). Ticks sourced from Utrecht center for Tick-borne diseases were used for the allocation of dogs to treatment groups (infestation on Day-7) and to infest dogs of the 3 groups on Day-2 and on Day 7, while ticks sourced from Biology Centre ASCR were used to infest dogs of the three groups on Day 28.
Group 1 dogs remained untreated while dogs from Groups 2 and 3 were treated once orally with NexGardÒ or NexGard SpectraÒ respectively. Chewables were chosen or combined as appropriate in order to dose the dogs, as close as possible, to the minimum effective dose of 2.5 mg afoxolaner per kg body weight.
Dogs were observed hourly for four hours post-treatment for adverse reaction to treatment. No drug was lost and no dog vomited.
On Day 2 (~48 hours after treatment), all ticks were removed and counted to evaluate the curative/immediate efficacy of afoxolaner for both products.
Subsequent tick removals and counts were made on Days 9, and 30 (each time, ~48 hours after infestation) to assess the persistent/sustained efficacy of the tested products.
In order to perform the tick counts, dogs were sedated using the same procedure as described for the infestation phases, and the whole body of each dog was examined for ticks. Attached ticks were removed using a tick extractor, and each tick was observed for signs of viability or mortality. The numbers of live attached, live free, dead attached, and dead free ticks was recorded. As male Ixodes ticks do not attach and feed on blood, the efficacy analysis was therefore based on the counts of attached female ticks.
2.4. Data Analysis
To evaluate the afoxolaner curative—immediate/therapeutic efficacy (efficacy against Day-2 Ixodes hexagonus infestations) and its persistent—sustained/preventive efficacy (efficacy against Day 7 and Day 28 infestations), arithmetic means of the live attached female tick counts were calculated by group at each time point. Percent effectiveness for each treated group was calculated using the formula:
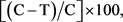
where C = arithmetic mean for the control group and T = arithmetic mean for the treated group.
To compare the groups, the natural logarithm of (counts + 1) was analyzed. The log-counts of each treated group was compared to the log-counts of the control group using an F-test adjusted for the allocation blocks used to randomize the animals to the treatment groups at each time point separately. The MIXED procedure in SAS Version 9.4 was used for the analysis, with the group listed as a fixed effect and the allocation blocks listed as a random effect. All statistical comparisons were two-sided at the 0.05 significance level.
3. Results
3.1. Health Observations
The animals were observed at least once daily for general health over the whole duration of the experimental phase. Following treatment, dogs were also observed hourly for 4 hours for adverse reactions to treatment.
On Day 5, one dog in Group 3 (NexGard SpectraÒ treated) engaged in an altercation with the two other dogs in the same cage and received a traumatic injury in the area of the cervical spine. This dog showed neurological signs, which was attributed to this injury. The animal was euthanized per the veterinarian recommendation, and the necropsy confirmed traumatic lesions in the cervical spine.
There were no adverse events related to treatment observed throughout the study, including during hourly observations conducted for 4 hours immediately after treatment.
3.2. Efficacy
Individual retention rates of live attached female ticks on the control animals ranged from 30.0% to 87.5% (Mean: 54.1% - 66.3%), demonstrating adequate infestation conditions with a vigorous tick population.
The percentage of efficacy of afoxolaner, using arithmetic means, in NexGardÒ group (Group 2) compared to the untreated control group was 100%, 100% and 96.7% on Days 2, 9 and 30, respectively. The percent efficacy of afoxolaner, using arithmetic means, in NexGard SpectraÒ group (Group 3) compared to the untreated control group was 100% at all post-treatment counts. There were significant differences in the population means between the treated groups and control group at all time points (p < 0.0001).
The analysis of the results is summarized in Table 1.
![]()
Table 1. Summary of analysis results of live attached female tick counts.
1Group 1 = Untreated Control; Group 2 = NexGardÒ; Group 3 = NexGard SpectraÒ; 2AM = tick arithmetic mean; 3n = number of animals; 4Percent efficacy = [(C − T)/C] × 100, where C and T are arithmetic means of Group 1 and Group 2 counts, or arithmetic means of Group 1 and Group 3 counts respectively; 5p-value = two-sided probability value from analysis of variance on log-counts of Group 2 and Group 1 or log-counts of Group 3 and Group 1.
4. Discussion and Conclusion
This experimental dose confirmation study was conducted to assess the efficacy of afoxolaner (NexGardÒ or NexGard SpectraÒ chewables) against Ixodes hexagonus, commonly called the hedgehog tick.
NexGardÒ is an afoxolaner-based palatable formulation marketed for the monthly treatment of fleas, ticks, demodicosis and sarcoptic mange in dogs. In the USA, NexGardÒ is also indicated for the prevention of Borrelia burgdorferi infections as a direct result of killing Ixodes scapularis vector ticks. NexGard SpectraÒ chewables for dogs have been developed as a convenient option for pet owners and veterinarians to treat and control both ecto and endoparasitic infections in dogs using a combination of afoxolaner and milbemycin oxime in a single chewable tablet.
Milbemycin oxime administered to dogs orally is used in several marketed formulations against adult intestinal nematode infections and to prevent heartworm disease. Given orally at a dose of 0.5 mg per kg body weight, this macrocyclic lactone does not demonstrate any activity against fleas and ticks in dogs, as published by Snyder and Wiseman in 2012 [31] ; and as shown in some studies conducted during NexGard SpectraÒ development (Merial, EMA registration dossier).
To assess afoxolaner’s efficacy against Ixodes hexagonus, three groups of dogs were used. Two groups of dogs were treated with afoxolaner alone (NexGardÒ) or combined with milbemycin oxime (NexGard SpectraÒ), both at a dose as close as possible to the minimum effective dose of 2.5 mg/kg afoxolaner. The third untreated group served as the negative control group to assess efficacy for both products.
In this study, tick challenges were not performed weekly, but on three occasions (Days-2, 7 and 28). The limited number of infestations nevertheless allowed the evaluation of afoxolaner’s curative efficacy against existing ticks, as well as its effectiveness against re-infestations both at the beginning and at the end of the month following treatment.
Despite the loss of two dogs in the NexGard SpectraÒ treated group due to reasons unrelated to treatment, this blinded, randomized, negative controlled clinical efficacy study met the requirements of international guidelines for ectoparasiticide efficacy testing in terms of sample size (at least 6 dogs per group), design and adequate strength of the tick challenge to provide statistically significant results.
The efficacy of a single treatment with afoxolaner, either alone or combined with milbemycin oxime resulted in 100% curative/immediate efficacy against I. hexagonus pre-existing infestations.
Afoxolaner in both products was also highly effective (96.7% to 100% efficacy) against re-infestations for at least 28 days after treatment.
The similar results obtained for afoxolaner in both formulations confirm the clinical equivalence of the two products regarding their efficacy against I. hexagonus ticks, as it has been previously demonstrated against Dermacentor reticulatus ticks [26] .
These results are consistent with previous experimental efficacy data published for afoxolaner against several other Ixodes species, e.g. Ixodes ricinus (98.5% - 100% efficacy for 35 days) [22], Ixodes scapularis (94.2% - 100% efficacy for 28 days) [24] and Ixodes holocyclus (97.7% - 100% efficacy for 35 days) [28] .
Ixodes hexagonus ticks thus seem to show the same level of susceptibility to afoxolaner as other Ixodes species.
The safety and efficacy of afoxolaner alone or in combination with milbemycin oxime observed in the present experimental study are also in accordance with the results of the pivotal multicentric field trials conducted in Europe for both products. In these 30-day field studies, treatment with NexGardÒ (afoxolaner) and NexGard SpectraÒ (afoxolaner plus milbemycin oxime) chewables consistently demonstrated > 98% and >95% efficacy respectively, against natural challenges with ticks including Dermacentor reticulatus, Ixodes ricinus and Rhipicephalus sanguineus [32], and Haemaphysalis concinna, D. reticulatus, I. hexagonus, I. ricinus, and R. sanguineus [27] [33] .
In conclusion, afoxolaner administered once orally at the minimum recommended dose in NexGardÒ or in NexGard SpectraÒ chewables is safe and highly effective against both existing and new I. hexagonus infestations in dogs for 4 weeks.
Acknowledgements
The authors would like to thank all the study personnel at CRSV for their technical assistance and Stephen Yoon for statistical analysis.
Funding
This clinical study was funded by Boehringer Ingelheim Animal Health. Wilfried Lebon, Marielle Servonnet, Diane Larsen, Pascal Dumont and Frederic Beugnet are employees of Boehringer Ingelheim Animal Health.