1. Introduction
Puccinia striiformis Westend. is an obligate biotrophic basidiomycete fungus. The species can be separated into different formae speciales based on host adaptation. For example, Puccinia striiformis f. sp. tritici (Pst) causes stripe (yellow) rust on wheat, which is a constraint for wheat production worldwide [1] [2] . Barley stripe rust, caused by P. striiformis f. sp. hordei (Psh), also can cause significant yield losses in many barley-growing regions [3] . Both formae speciales also can infect and grow on many wild grasses [4] [5] . To better manage stripe rust on cereal crops, it is important to understand the biology of this pathogen and its adaptation to different host plants and climates.
Like many rust species, P. striiformis is a heteroecious, macrocyclic fungus with five spore stages. The fungus produces yellow-orange colored uredinia and black telia on its primary hosts of cereal crops and auxiliary hosts of wild grasses. Urediniospores are infectious and produced for many generations. It is the uredinial stage that causes disease epidemics. After uredinial formation, telia may form to replace uredinia. Teliospores produced in telia are not infectious themselves, but produce basidiospores if germinate. Basidiospores infect alternate hosts, on which pycniospores (spermatia) are produced on the adaxial surfaces of leaves and after fertilization of receptacle hyphae by pycniospores, aeciospores are produced on the abaxial surfaces directly from the fertilized pycinia [6] [7] .
For P. striiformis, alternate hosts were not known until 2010 when barberry was found to be infected under natural conditions by the bluegrass stripe rust pathogen, P. pseudostriiformis (previously P. striiformis f. sp. poae) and under controlled conditions by the wheat stripe rust pathogen Pst [8] . Infections of barberry by Pst were found at very low frequencies under natural conditions in China [9] [10] . However, aecia on barberry plants in the US Pacific Northwest were all identified as Puccinia graminis (the stem rust pathogen), but no P. striiformis aecia were detected [11] . Wang and Chen [12] identified four factors that prevent barberry from being infected by P. striiformis, including the fact that teliospores are enclosed in epidermis-covered telia and lack of dormancy, degradation of teliospores under the wet winter conditions, and phenology mismatch between barberry plants and P. striiformis teliospores.
The telial stage is the connection between the asexual cycle and sexual cycle for a macrocyclic rust fungus. Telial formation is a switch by the rust pathogen in response to the unfavorable conditions of host plants and environments to prepare for survival. Telia of P. striiformis usually form when leaves mature or are dried by urediniospore production and when the weather is hot. In the Pacific Northwest, telia of P. striiformis are usually observed after host plants flowering, normally from June for winter crops and from July for spring crops of wheat and barley. However, in recent years, telia were observed in early growth stages and seasons when leaves were still green. When the barley stripe rust first appeared in the United States in the early 1990s [3] , it was noticed that barley stripe rust isolates produced telia on barley plants under the greenhouse conditions ideal for producing uredinia, but wheat stripe rust isolates did not produce telia. Since 2017, it also has been noticed that a group of races, represented by PST-127 and PST-139 based on the old cultivar differentials [13] or PSTv-11 and PSTv-14 based on the new Yr single-gene differentials [14] [15] , more readily produce telia than other race groups under the same controlled greenhouse conditions and in the fields. Thus, an isolate of this race group was used to develop a sexual population for mapping Pst avirulence genes [16] . Similarly, Rodriguez-Algaba [17] selected a Danish isolate to obtain sexually reproduced isolates based on its ability to produce abundant teliospores under both greenhouse and field conditions. Tian et al. [18] and Wang et al. [19] also used Chinese Pst isolates that produce abundant telia to develop sexual populations for studying genetics of virulence. Telial formation is the precondition for sexual reproduction. Without telia, infection of alternate hosts and sexual reproduction are not possible.
However, telial formation does not always lead to sexual reproduction on alternate hosts depending upon telial structure, climatic conditions, and the phenology of alternate hosts [7] [12] . If a rust pathogen is not able to infect alternate hosts, telial formation may be a disadvantage in aggressiveness and fitness as it stops urediniospore production. Ali et al. [20] tested 56 Pst isolates from northern France, the Mediterranean region, the Middle-East, Pakistan, Nepal, China, and Denmark for their ability to produce telia. They found that isolates from China, Nepal, and Pakistan displayed the highest telial production, while clonal populations from France and the Mediterranean region displayed very low telial production. This study did not include any isolates from the United States except two Danish isolates that were considered as the recently spread US-European clone.
The stripe rust pathogen is unable to infected barberry under the natural conditions in the Pacific Northwest of the United States, although the pathogen population is able to produce telia in the fields [11] [12] . Identification of the variation in telial formation among P. striiformis isolates may help understand the pathogen evolutions, and such data may be useful in predicting population changes. Therefore, the objectives of this study are to determine variation in telial formation among P. striiformis isolates in the United States and identify possible factors affecting telial production.
2. Materials and Methods
2.1. Stripe Rust Sample Collection
Stripe rust was surveyed by the authors in the Pacific Northwest and by collaborators throughout the United States during the wheat growing seasons in 2013-2016. During the disease monitoring surveys, leaves bearing P. striiformis uredinia were collected from wheat, barley, triticale, rye, and various grasses. In general, one or more leaves with uredinia from a cultivar, germplasm, or breeding line in a field were treated as a single sample. When a sample was collected, collection date and location (city, county, and state), host plant, cultivar name or line number, plant growth stage, rust prevalence (percentage of plants infected in the cultivar field or for the entry in a nursery) and severity (percentage of leaf area infected), and infection type were recorded whenever possible. Samples were kept dry at 4˚C before being used for inoculation.
2.2. Increasing Urediniospores
Fresh urediniospores were obtained for each sample by inoculating seedlings following the previously described procedure [14] [21] [22] . Samples from wheat were used to inoculate wheat cultivar Nugaines as it is susceptible to all Pst races identified thus far in the United States [14] [15] [22] [23] . Samples from barley were used to inoculate barley cultivar Steptoe as it is susceptible to all Psh races [3] [22] [23] . Samples from triticale, rye, and grasses were used to inoculate both Nugaines and Steptoe as the rusts could be either Pst or Psh [5] . Briefly, two-leaf stage seedlings were inoculated with urediniospores from collected samples. Inoculated plants were incubated in a dew chamber at 10˚C for 24 h without light and then kept in a growth chamber with a diurnal temperature cycle changing from 4˚C at 2:00 am to 20˚C at 2:00 pm with 16 h light. Inoculated plants with urediniospores from individual samples were separated using a booth to avoid contamination. Urediniospores were collected and stored in a desiccator at 4˚C. Race identification for each isolate was conducted using the Yr single-gene line differentials and the standard procedure as previously described [14] [15] .
2.3. Recording Telial Formation
After preliminary experiments using several susceptible varieties, spring wheat “Avocet S” (AvS), which is susceptible to almost all Pst races, was selected for recording telial formation of Pst isolates, and Steptoe was selected for recording telial formation of Psh isolates 40 days after inoculation. Two-leaf seedlings of AvS or Steptoe grown in a plastic pot of 7 × 7 × 7 cm were uniformly inoculated using fresh urediniospores of an isolate collected from Nugaines or Steptoe, as mentioned above, mixed with talcum at a 1:20 ratio [14] . Inoculation and growth of the plants after inoculation were the same as described above. Based on the preliminary experiments, 40-day after inoculation was found to be the best time for scoring the percentage of telial formation, and therefore, used to recording telial formation. For each isolate, the inoculation of AvS or Steptoe was done at the same time when testing on the differentials for race identification. The infection type of the uredinial stage was scored at 19 - 21 days after inoculation [14] , whereas the plants of AvS were kept at the same temperature (diurnal temperature cycle of 4˚C - 20˚C) and light (16 h daily photoperiod) cycles until 40 days after inoculation for scoring telial formation. The percentage of telia was recorded by visual assessment based on the scale of 0 (no telia) to 100% (telia occupying all sporulated areas) (Supplementary Figure 1).
2.4. Data Analysis
The isolates were classified based on year, month, state or epidemiological regions, host plants from which they were collected, and race. Based on wheat cropping systems, geographic barriers, and spore movement of the stripe rust pathogen, the inland United States is separated into 12 epidemiological regions as the following: region 1 (R1) includes eastern Washington, northeastern Oregon, and northern Idaho; R2 includes western Montana; R3 includes southern ID, southeastern Oregon, northern Nevada, northern Utah, western Wyoming, and western Colorado; R4 includes western Oregon and northern California; R5 includes northwestern Washington; R6 includes central and southern California, and Arizona; R7 includes Texas, Louisiana, Arkansas, Oklahoma, and eastern New Mexico; R8 includes Kansas, Nebraska, and eastern Colorado; R9 = South Dakota, North Dakota, and Minnesota; R10 includes Mississippi, Alabama, Florida, Georgia, South Dakota, North Dakota, Tennessee, and Kentucky; R11 includes Missouri, Illinois, Indiana, Iowa, Wisconsin, and Michigan; and R12 includes Virginia, West Virginia, Ohio, Maryland, Pennsylvania, and New York [13] . The PROC MIXED procedure was used in the SAS for windows (Version 9.2, SAS Institute, Cary, NC) to determine the effects of the classified parameters and their interactions on telial formation. The percentage of telial formation was used as the dependent variable; and year, month, epidemiological region (or state), host, and race as independent variables.
3. Results
3.1. Telial Formation
Under the test conditions, uredinia started appearing approximately 14 days after inoculation and telia started appearing 25 days after inoculation on the inoculated leaves. As the time was progressing, more telia appeared and more uredinia disappeared. However, significant differences existed among the isolates in the speed of pustules converting from uredinia to telia. As the objective of this study was to find differences in telial formation among isolates, we did not monitor the progression of uredinial conversion to telial for all isolates, but set the 40 days after inoculation as the time to scoring the percentage of telial formation. The minimum, maximum, and mean percentages of telial formation, as well as the number of isolates, based on each of year, month, epidemiological region, host, and race are presented in Table 1. Of the 1423 isolates, 527 (37.03%) did not produce any telia, whereas the remaining 896 (62.97%) produced telia (Figure 1). However, the percentage of telial formation varied dramatically among the isolates producing telia, ranging from 1% to 95%. Isolates producing trace amount (1% - 10%) of telia counted for 25.51%, those producing light amount (11% - 30%) of telia 14.97%, those producing intermediate amount (31% - 70%) of telia 16.92%, and those producing high amount (71% - 95%) for 4.56% of the 1423 isolates. The frequencies of different levels of telial formation varied from year to year. The populations of 2013 and 2015 produced more telia than those of 2014 and 2016. A high proportion of isolates in 2016 (18.55% of the total and 76.52% of the 2016 isolates) did not produce telia, more than any other years (Figure 1).
![]()
![]()
Table 1. Telial production of Puccinia striiformis isolates in different years (2015-2016), months, epidemiological regions, and plant hosts.
aSD = standard deviation. bP = probability. cNA = not applicable. dThe 12 epidemiological regions of the inland United States are: Region 1 (R1) = eastern Washington, northeastern Oregon, and northern Idaho; R2 = western Montana; R3 = southern ID, southeastern Oregon, northern Nevada, northern Utah, western Wyoming, and western Colorado; R4 = western Oregon and northern California; R5 = northwestern Washington; R6 = central and southern California, and Arizona; R7 = Texas, Louisiana, Arkansas, Oklahoma, and eastern New Mexico; R8 = Kansas, Nebraska, and eastern Colorado; R9 = South Dakota, North Dakota, Minnesota, and eastern Montana; R10 = Mississippi, Alabama, Florida, Georgia, South Dakota, North Dakota, Tennessee, and Kentucky; R11 = Missouri, Illinois, Indiana, Iowa, Wisconsin, and Michigan; and R12 = Virginia, West Virginia, Ohio, Maryland, Pennsylvania, and New York [13] . eInclude 7 isolates from triticale and 1 isolate from rye. fraces having 1-4 isolates.
![]()
Figure 1. Frequency distribution of telial formation in 1423 isolates of Puccinia striiformis collected in 2013-2016.
3.2. Factors Significantly Affecting Telial Formation
The mixed model analysis identified that parameters year (P < 0.0001) and month (P = 0.0004) were significantly associated to telial formation, race was marginally associated (P = 0.0984), whereas epidemiological region (P = 0.1528) and host (P = 0.6530) did not contribute significantly to the telial variation (Table 2). In addition, interactions between region and month (P = 0.008), year and region (P = 0.0111), year and race (P = 0.0539), and month and region (P = 0.0039), as well as the interaction among year, month, and region (P < 0.0001), were significantly affected telial formation. The interactions between month and race (P = 0.0921) and among year, month, and race (P = 0.0971) were marginally affected telial formation. As telial formation varied significantly among isolates collected in different months, epidemiological regions, and races in different years, the differences in telial formation will be presented by month, region, and races across 2013-2016 in the following paragraphs.
3.3. Telial Formation in Isolates Collected from Different Years and Months
In general, isolates collected in 2013 and 2015 tended producing more telia than those collected in 2014 and 2016 (Figure 1). Within each year, isolates collected in different months had different percentages of telial formation (Figure 2). For example, isolates collected in May and June produced more telia than those collected in earlier or later months in 2013. However, in 2015, isolates collected in April had the highest telial formation. In both 2014 and 2016, isolates collected in the earliest months (March in 2014 and February in 2016) produced more telia than those collected in other months.
3.4. Telial Formation in Isolates Collected in Different Epidemiological Regions
In 2013, the highest telial formation was observed in isolates collected from Region 2 (R2, western Montana) and the lowest from R4 (western Oregon) (Figure 3). In 2014, higher telial formation was observed in isolates collected from R7 (south-central Great Plains) than those from R2 - R4 and R8 - R12. In 2015, isolates of R10 (southeastern states) and R12 (northeastern states) produced more telia than those from other regions. In 2016, the mean telial formations of R5 - R7 were relatively high compared to those of other regions, but not statistically different from other regions as the telial formation was generally low and the variation was high among the isolates collected in these regions.
![]()
Table 2. Effects of year, race, region, month, and host on the telial production of Puccinia striiformis.
aP = probability. *denotes significance at P = 0.05; **0.05 > P > 0.0001; and *** P < 0.0001. b - indicates no value.
![]()
Figure 2. Telial formation of Puccinia striiformis in different months of 2013-2016.
![]()
Figure 3. Telial formation of Puccinia striiformis in different epidemiological regions in 2013-2016 (see the footnote of Table 1 for epidemiological regions).
3.5. Telial Formation among Isolates of Different Races
Similar to months and epidemiological regions, isolates in each race varied in telial formation within a year and also from year to year. In 2013, isolates of Pst race PSTv-53 had the highest mean telial formation, which was significantly higher than those of Psh race PSH-33 and Pst races PSTv-18 and PSTv-48 (Figure 4). In 2014, PSTv-53 was produced more telia than other races. In 2015, races Psh races PSH-33 and PSH-48 and Pst races PSTv-4, PSTv-11, and PSTv-53 had significantly higher telial formation than PSTv-18, PSTv-37, and PSTv-48. In 2016, race PSH-48 had higher telial formation than any of the races shown in Figure 4, but not significantly different from race PSH-33.
3.6. Interactions among Different Years, Months, and Regions
As the interaction among the different years, months, and regions (Table 2) was a significant contributor to the variation in telial formation, the minimum, maximum, and mean percentages of telial formation, as well as the numbers of isolates, in each category of these parameters are given in Supplementary Table 1. For visual comparison, the mean percentages of telial formation for the categories with 10 or more isolates are presented in Figure 5. The majority of the categories had a wide range of telial formation. However, some of the categories had significantly high telial formation than others. For example, isolates collected from R10 in May, and R8 and R11 in June of 2013; and R1, R6, and R7 in April and May, and R1 in June of 2015 had higher telial formation than many other regions of other months and years. In general, isolates collected in 2014 and 2016 in different months and from different regions had lower telial formation than those in 2013 and 2015. Isolates collected from R10 in April and R12 in June of 2016 did not produce any telia.
![]()
Figure 4. Telial formation of different Puccinia striiformis races in 2013-2016.
![]()
Figure 5. Telial formation of Puccinia striiformis isolates (≥10 isolates in each combination) in different regions and months of 2013-2016.
4. Discussion
Telial formation is a complex trait for rust fungi. For P. striiformis, telia did not catch much attention until the recent discovery that the species is able to infect barberry and mahonia [6] [7] [8] . In the present study, we found tremendous variation in telial formation among isolates of P. striiformis in the United States. This result was similar to the variation in telial production among Pst isolates in other countries [20] . In the present study, the variation was greatly associated to the month and year when the isolates were collected, and to a less extend, related to the races of isolates. Interestingly, host did not contribute significantly to the observed variation in telial formation. Thus, telial formation is a genetic trait greatly under the influence of environmental conditions, but independent of host adaptation and to a less extent, independent of virulence patterns.
Telia, which contain teliospores, are the last fruiting structure of the rust fungi during their lifecycle. Following urediniospore production, teliospores are formed as a secondary type of spores in uredinia. The uredinia are thus transformed into telia. Depending upon rust species, telia may be open or covered by the epidermis of the host plants. Telia of P. striiformis are mostly under the epidermis of the host plants, and thus breaking the epidermis layer is needed to release and germinate teliospores [12] . The observations in the present study confirmed the previous report.
Telia of macrocyclic rust fungi are generally formed late in the season [24] . We observed that Pst telia are more easily produced on leaves of adult-plant (data not shown). For the purpose of producing a large amount of telia, adult plants were inoculated with urediniospores, and when sporulation reached to a heavy level, the temperature was increased from constant 16˚C to 25˚C for the daytime to speed up telial formation [18] . However, telia of Puccinia triticina, the wheat leaf rust pathogen, are also produced on young plants [25] . Similarly, we observed Pst telia on seedlings of wheat under both controlled greenhouse and field conditions. Indeed, we conducted the present study entirely on seedlings in the greenhouse to reduce the space and shorten the time needed for testing a large number of isolates. The seedling testing worked well for distinguishing isolates with different capabilities of producing telia.
Previous studies attempted to determine the physiological background of telial formation. As discussed above, plant growth stage and temperature are definitely related to telial formation, but not always, as shown by the present study. Many early studies have shown that telial formation in different cereal rust fungi correlates with the cultivar-race combination [25] - [36] . We also observed differences in telial formation in isolate-cultivar combinations in our preliminary tests. On the differentials in our race identification, some isolates produced telial on differentials showing moderate infection types than those of highly susceptible (data not shown). As our purpose was to determine the difference in telial formation among the large number of isolates, we only tested the Pst isolates on AvS and Psh isolates on Steptoe. Further studies are needed to test some of the isolates selected based on the results of the present study on different cultivars for determining the interactions between isolates and cultivars.
Telial formation is isolate-dependent. In the present study, we identified 37% of the tested isolates did not form telia under the testing conditions, whereas the remaining 63% isolates produced telia ranging from 1% to 95%. The variation appeared not significantly correlated to races as isolates identified as the same race also had a wide range of telial formation. Our results were consistent with some previous studies on other rust fungi. Working with oat crown rust, Zimmer and Shafer [36] reported that rapidity of telial formation was not correlated with range of virulence, specific virulence of the pathogen, or with maturity of the host. They observed that some isolates did not produce telia under any of the testing conditions. Their study suggested that some isolates may have lost the capacity to produce telia under any conditions. Both their study and the present study indicated that different isolates may have different genes involving in telial formation, and the expression of these genes may under the influence of different environments and host conditions. Further work is needed to identify these rust genes and determine how they respond to different host conditions and environments.
A previous study reported association between molecular genotypes with telium-producing race groups [37] . In that study, Pst isolates were mostly collected from the U. S. Pacific Northwest (R1 in this study), and the majority of isolates belonged to molecular group (MG) 1, with predominantly homozygous loci. The isolates in MG 1 were in the virulence group (VG) 1, with a wider virulence pattern, especially virulence to resistance genes Yr1, YrSP, and Yr76 (YrTye). The correlation of the molecular group with the virulence group was consistent with another study that PSTv-11 and PSTv-14 (previously PST-127 or PST-139) possess more homozygous simple sequence repeat (SSR) loci than other races [5] . Similarly, Ali et al. [20] reported that certain Pst genotypes highly produce telia, especially isolates from China, Nepal, and Pakistan. In the U. S. PNW, the Pst population generally did not produce abundant telia until the appearance of PST-127 and related races in MG 1, which was first identified in California and Washington in 2007 and became predominant in the western United States in 2009-2012 [13] [14] [15] [22] . This group of races produces abundant telia in the fields and also on adult plants under controlled greenhouse conditions. Since 2013 (the beginning of the present study), this race group has decreased dramatically in the western United States (A. M. Wan, M. N. Wang, and X. M. Chen, unpublished data). PSTv-11 and PSTv-14 were detected at low frequencies in the eastern United States (R7, R8, and R11) only in 2012 with exceptions of one isolate of PSTv-14 in R7 (and also one isolate of PSTv-14 in Ontario, Canada in 2017) (M. N. Wang and X. M. Chen, unpublished data). Similarly, Brar et al. [38] identified two lineages (PstPr and PstS1-related) from western Canada, which produced more telia than other lineages. Based on the virulence data, the isolate of PSTPr, collected in 2011, was similar to races PSTv-11 and PSTv-14. The increase of the race group in the U. S. Pacific Northwest from 2007 to 2012 could be attributed to its wide spectrum of virulence, which overcomes resistance genes like Yr1, Yr17, Yr43, Yr44, YrTr1, YrExp2, and Yr76 (YrTye) in wheat cultivars grown in this region [14] [15] . The widely used non-race specific, but partial high-temperature adult-plant (HTAP) resistance in most of the cultivars grown in the Pacific Northwest has allowed this group of races growing to some extent. The relative restriction in distribution and the decrease of this race group could be related to the high formation of telia.
Telial formation is a double-edged sword for rust fungi, especially for P. striiformis. Quick formation of telia stops producing infectious urediniospores to save energy and life for surviving unfavorable climate conditions. This is an advantage if the fungus has essential sexual production in their lifecycle, such as P. graminis in the U. S. Pacific Northwest [11] [12] . Telial formation occurs just few days after the appearance of uredinia in the field, usually in July and the late plant growth stages. When the uredinial stage cannot overwinter, the pathogen must produce from barberry infection by basidiospores from teliospores to infect cereal crops. However, P. striiformis can survive at the uredinial stage all year long in this region. Its telia cannot survive the wet winter [12] . So far, natural infection on barberry by P. striiformis has been reported at very low frequency in China [7] [9] [10] and by a similar species (P. pseudostriiformis) in Minnesota, the United States [8] . In many other wheat and barley growing regions, including the United States and Europe, P. striiformis has not been found on barberry [7] [11] [39] . When telia do not have the function for survival and subsequent producing infectious basidiospores to infect alternate hosts, telial formation is a suicide, which puts isolates with quick telial formation into a disadvantageous position for less competitive against other isolates. Thus, high telium-producing isolates are likely less aggressive, less fit, and eventually replaced by those not quickly producing telia. Before the PSTv-11 race group reached its peak frequency, we predicted its decrease based on the high production of telia, although its advantage in cultivar adaptation. As discussed above, the data so far has proven the hypothesis. In the present study, we presented four-year telial formation data, and the last year (2016) of the period had much lower telial production than the previous three years. It will be useful to continue recording telial formation of new isolates or populations to prove the hypothesis that telial formation is disadvantageous for P. striiformis. Such data, as well as the data of the present study, may be used to predict relative fitness and aggressiveness of different races or populations, and in turn, the information may be used to select effective resistance genes for developing durable resistant cultivars. At the same time, more studies are needed to determine the genetic basis of telial formation and its regulation by the different environment conditions.
5. Conclusion
Telial formation is a stage during the stripe rust pathogen lifecycle to switch the fungus from producing urediniospores for infecting cereal and grass hosts to producing teliospores for survival. It connects the asexual and sexual cycles. Under the climatic conditions and alternate host phenology that are unfavorable to the survival of telia and infection of alternate host, telial formation functions as self-killing and reduces fitness and aggressiveness. In the present study, we found that the percentages of telial formation varied greatly among the pathogen isolates, and the formation of telia was significantly affected by the year and the month when the isolates were collected. In contrast, the epidemiological regions or states, host plants, and races did not significantly affect telial formation. However, significant effects on telial formation were observed by interactions between year and region, year and race, month and region and among year, month, and region, as well as between year and month. These results indicate that telial formation is a complex trait under the genetic control of the pathogen isolates for adaptation to different environments. Further studies are needed to identify genes involved in the formation of telia and the relationship of telial formation to the survival, aggressiveness, fitness, and evolution of the pathogen.
Acknowledgements
This research was supported by the U.S. Department of Agriculture, Agricultural Research Service (Project No. 2090-22000-018-00D), Washington Grain Commission, Idaho Wheat Commission. Department of Plant Pathology, College of Agricultural, Human, and Natural Resource Sciences, Agricultural Research Center, Project No. WNP00663 (Projects 13C-3061-5665 and 13C-3061-4232), Washington State University, Pullman, WA 99164-6430, USA. We thank the following collaborators for collecting and sending stripe rust samples in 2013, 2014, 2015, and 2016: Zewdie Abate, Maricelis Acevedo, Robert Allan, Tom Allen, Kelly Arceneaux, Mark Avery, Adrian Barta, Araby Belcher, Gary Bergetrom, Tamla Blunt, Bob Bowden, Kira Bowen, Carl Bradley, Matt Breiland, Kirk Broders, Mary Burrows, Emmanuel Byamukama, Kim Campbell, Oly Cantu, Arron Carter, Connie Cawthon, Jay Chapin, Nicolas Cobo, Kevin Cochrane, John Coffey, Christina Cowger, Jaime Cummings, Heather Darby, Patricia DeMacon, Eric DeWolf, Frank Dugan, Kent Evans, Michael Flowers, Barton Fogleman, Andrew Friskop, Michael Fulcher, Sam Gale, John Garner, Chuck Gatzmeier, Polly Goldman, Tyler Gordon, Carl Griffey, Scott Haley, Samantha Hardin, Stephen Harrison, Pat Hayes, Jud Hedine, Christina Hegarty, Joshua Hegarty, Harua Helgerson, Don Hershman, Paul Hobson, David Hole, Scott Hulbert, Bob Hunger, Amir Ibrahim, Chad Jackson, Lee Jackson, Yue Jin, Heather Kelly, Nathan Kleczewski, Mathias Kolding, Mark Larson, Melissa Lim, David Long, Shari Lupien, Juliet Marshall, Pete Martin, Phil Mayo, Jeff Millen, Eugene Milus, David Moon, Jacki Morrison, Brian Mueller, Ron Muzzana, Justin O'dea, Eric Olson, Lucinda Olson, Boyd Padgett, Stine Peterson, Bob Pitman, Jill Pollok, Bruce Potter, Paul Price, Trey Price, Mike Pumphrey, Shag Richter, J. Riddle, Roland Sears, Elda Serec, Paul Severns, Linnea Skogland, Damon Smith, Jacqueline Smith, Madeleine Smith, Bryan Simoneaux, Jason Spooty, Trent Stanger, Brian Steffenson, Bob Stougaard, William Stump, Albert Tenuta, Mandy Tolbert, Nick Troy, Claudia Vischwifz, Stephen Wegulo, Andrew Wiersma, Greg Williams, Kiersten Wise, Kay Wood, Chao Xiang, John Youmans, Bob Zemetra, and Junli Zhang.
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U. S. Department of Agriculture. USDA is an equal opportunity provider and employer.
This article is in the public domain and not copyrightable. It may be freely reprinted with customary crediting of the source American Journal of Plant Sciences.
Supplementary Files
Supplementary Table 1. Telial formation of Puccinia striiformis isolates grouped by year, month, and epidemiological region of collection.
aSee the footnotes of Table 1 for epidemiological regions. bSD = standard deviation. cNA = not applicable because of only one isolate.
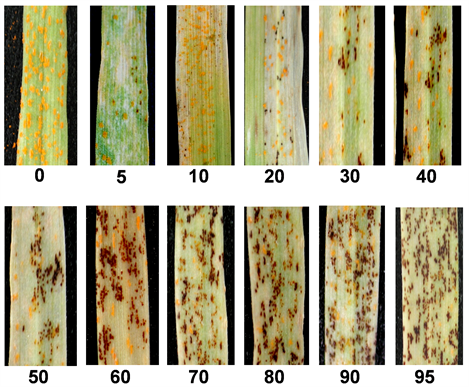
Supplementary Figure 1. Percentages of telial formation among Puccinia striiformis.