1. INTRODUCTION
Positron Emission Tomography (PET) and its related hybrid technologies such as PET/CT and PET/MR are non-invasive imaging modalities that provide in vivo physicochemical, pharmaco-kinetic information, measurement and quantification of biochemical processes [ 1 - 4 ]. The unique sensitivities of these techniques allow the detection of cellular changes during disease progression. PET imaging provides quantitative bio-distribution data of molecular probes. Positron annihilation by electron releases two antiparallel γ-photons that are detected simultaneously by γ-cameras and used to construct the PET images. Currently PET is used clinically to detect cancers [ 5 , 6 ], cardiovascular diseases, and neurological disorders [ 7 ]. Also PET has become an important tool in drug discovery and development [ 8 ]. Of several short lived β+-emitting (positron) nuclides available, the most attractive is fluorine-18 because of its low energy, moderate half-life (109.6 min), and its easy availability in “no-carrier-added” form. During the past decade, various transition-metal-mediated [ 9 - 12 ] (Ag, Pd, Ni, Cu) fluorination methods have been transferred to radiochemistry. Among these, Cu-promoted [ 13 - 16 ] fluorination methods are the most versatile (Cu is less toxic compared to Pd and Ni) for preparing 18F-fluorinated aromatics and hetero aromatics (Figure 1).
2. MATERIALS AND METHODS
All reagents and solvents were purchased from Acros or Aldrich and were used as received.
![]()
Figure 1. Copper catalyzed radio-fluorinations of arenes.
Flash column chromatography was performed using silica gel (60 A, 230 - 400 mesh, Sorbent Technologies, USA) (Still, 1975). Analytical thin-layer chromatography (TLC) was performed using 250 μm silica plates (Analtech, Inc., Newark, DE) with a visualization by UV (254 nm) or phosphomolebdic acid spray. 1H and 13C-nuclear magnetic resonance spectra (NMR) were recorded at 300 or 125 MHz, respectively. Chemical shifts for 1H-NMR and 13C-NMR spectra were referenced to the residual protons of the deuterated solvents or to TMS. High resolution mass spectrometry was performed using a JEOL AccuTOF™ DART Mass Spectrometer. No-carrier-added [18F]F−, produced from recycled [18O] water, was obtained from PetNet (Knoxville, TN). Thin-layer chromatography visualization was performed with radiation detectors using a BioScan AR-2500 radio-TLC reader and Win Scan 1.3 software. Radio-TLC plates were developed using EtOAc/hexane (1/9). F-18 labelling was performed using Advion NanoTek Microfluidic Synthesis System controlled by NanoTek LF 1.4 Software.
4-[18F]fluoro-1,1’-biphenyl (4): Cyclotron-produced, no carrier added[18F]fluoride ion (ca. 1.85 GBq) in [18O] water (225 - 350 μL) was first adsorbed onto anion exchange resin ORTG cartridge within the concentrator module of a NanoTek System and released with a solution of K2CO3 (1.8 mg), kryptofix (12.0 mg) in MeCN/H2O (9.5:0.5 v/v, 400 μL) into 5 mL v-vial. The solution was dried by three cycles of azeotropic evaporation with MeCN (0.45 mL) at 100˚C.
To this anhydrous 18F−-K222-K+ complex was added the boronate ester 3 (16.7 mg, 0.06 mmol), and Cu(OTf)2(Py)4 (4.1 mg, 0.006 mmol) and DMF (0.40 mL). The resulting mixture was stirred at 110˚C for 20 min. The product was diluted with water (10 mL) and passed through C18 Sep-Pak to remove unreacted fluoride and the product was eluted with ethyl acetate (3 mL) to obtain 1.26 GBq of 4-[18F]fluoro-1, 1’-biphenyl (68% E.O.S). The radio-TLC was performed on silica gel plate using ethyl acetate:hexanes (1:9) as eluent.
Ethyl 4-chlorobenzoylglycinate (10): To a solution of 4-chlorobenzoyl chloride (1.65 g; 10.0 mmol), 8, triethyl amine (1.50 g; 15.0 mmol) in CH2Cl2 at 0˚C was added ethyl glycinate hydrochloride (1.68 g; 12.0 mmol), 9, in small portions over a 10 min. period. The temperature was slowly raised to room temperature and the mixture stirred for 3 hr. The solvent was removed and the residue was treated with anhydrous ether (20 mL) and separated triethyl amine hydrochloride was filtered off. The ether layer was thoroughly washed sequentially with water (10 mL), saturated solution of sodium bicarbonate (10 mL), brine (10 mL), and then dried over anhydrous sodium sulfate. Removal of the ether under reduced pressure yielded a colorless solid (2.29; 95%). 1H NMR CDCl3 (δ) 1.26 (t, 3H, CH3), 4.22 (q, 2H, CH2), 6.65 (bs, 1H, NH), 7.41 (d, J = 7 Hz, 2H, Ar-H2) and 7.69 (d, J = 7 Hz, 2H, Ar-H2).
Ethyl 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolyl)benzoylglycinate (11): To a flame-dried nitrogen flushed 25 ml round-bottomed flask were added Pd(OAc)2 (2.30 mg, 0.01 mmol), Gorlos-Phos-HBF4 (19.0 mg, 0.04 mmol), KOAc (0.29 g, 3.00 mmol), bis(pinacolato)diboron (0.31 g, 1.20 mmol), ethyl 4-chlorobenzoylglycinate (0.24 g, 1.00 mmol) and dioxane (2 mL). The flask was evacuated and back filled with nitrogen two times. The reaction mixture was heated for 2.0 hr at 90˚C and was diluted with ethyl acetate (10 mL) and filtered through a short column of silica gel (eluent: 2 × 10 mL of ethyl acetate). Evaporation and purification by column chromatography afforded ethyl 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolyl) benzoyl-glycinate (176.0 mg, 53%) as a colorless solid. 1H NMR CDCl3 (δ) 1.39 (t, 3H, CH3), 1.40 (s, 12H, 4 x CH3), 4.29 (s, 2H, CH2), 4.38 (q, 2H, CH2), 6.90 (bs, 1H, NH), 7.82 (d, J = 8.4 Hz, 2H, Ar-H2) and 7.95 (d, J = 8.4 Hz, 2H, Ar-H2). 13C NMR CDCl3 (δ) 24.9, 41.8, 61.7, 84.9, 124.4, 135.1, 137.3, 166.9 and 170.3. HRMS (ES) calculated for (M + H) C17H24BNO5: 334.18160. Found: 360.18289.
Ethyl 4-[18F]fluorobenzoylglycinate (17): The details of the NanoTek Microfluidic Synthesis System have been described previously [ 21 ]. Cyclotron-produced, no carrier added[18F]fluoride ion (ca. 1.85 GBq)) in [18O] water (225 - 350 μL) was first adsorbed onto anion exchange resin ORTG cartridge within the concentrator module of a NanoTek System and released with a solution of K2CO3 (1.8 mg), kryptofix (12.0 mg) in MeCN/H2O (9.5:0.5 v/v, 400 μL) into 5 mL.
v-vial. The solution was dried by three cycles of azeotropic evaporation with MeCN (0.45 mL) at 110 oC. To this anhydrous 18F−-K222-K+ complex was added the boronate ester 11 (20.0 mg, 0.06 mmol), Cu(OTf)2 (21.0 mg, 0.06 mmol) and Cu(OTf)2(Py)4, (4.1 mg, 0.006 mmol) and DMF (1.0 mL). The resulting mixture was stirred at 110˚C for 20 min. The product was diluted with water (10 mL) and passed through C18 Sep-Pak to remove unreacted fluoride and the product was eluted with ethyl acetate (3 mL) to obtain 0.89 GBq of ethyl 4-[18F]fluorobenzoyl glycinate (48% E.O.S).The radio-TLC was performed on silica gel plate using ethyl acetate:hexanes (1:9) as eluent.
4-Chlorophthalimide (13): A mixture of 5-chlorophthallic acid monosodium salt (1.99 g, 10.0 mmol) and ammonium carbonate (1.38 g, 14.3 mmol) was fused at 300 oC by pushing the sublimed material back into the flask intermittently. The molten product was quickly poured into an evaporating dish. The brownish solid was pulverized into a fine dust to obtain 4-chlorophthalimide, 13 (1.71 g, 95%). 1H NMR CDCl3 (δ) 7.65 (d, J = 7.8 Hz, 1H, Ar-H), 7.69 (d, J = 7.8 Hz, 1H, Ar-H) and 7.71 (s, 1H, Ar-H), 13C NMR CDCl3 (δ) 123.4, 124.6, 130.1, 133.9, 134.9, 141.6, 165.4. 166.3.
4-Chlorophthaloylglycinate (15): A mixture of 5-chlorophthalimide, 13, (348.2 mg, 1.93 mmol), ethyl bromoacetate (379.3 mg, 2.02 mmol), cesium carbonate (658.4 mg, 2.03 mmol) in anhydrous dimethyl formamide (5 mL) was stirred at room temperature for 2.5 h. the reaction mixture was poured into ice cold water and extracted with ether (2 × 25 mL). The combined ether extracts were washed with brine (10 mL) and dried over anhydrous sodium sulfate. Removal of the solvent provided a gummy material which was purified by using silica gel column chromatography (eluent: ethyl acetate:petroleum ether = 1:4) to obtain 15 as a colorless solid (448.0 mg, 87%). 1H NMR CDCl3 (δ) 1.28 (t, 3H, CH3), 4.23 (q, 2H, OCH2), 4.42 (s, 2H, NCH2), 7.69 (d, J = 8.4 Hz, 1H, Ar-H), 7.81 (d, J = 8.4 Hz, 1H, Ar-H) and 7.85 (s, 1H, Ar-H) 13C NMR CDCl3 (δ) 15.8, 39.7, 62.4, 123.8, 124.9, 130.1, 134.1, 134.9, 141.6, 165.8. 166.1 and 167.2.
Ethyl 3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolyl)phthaloylglycinate (16): To a flame-dried and nitrogen filled 25 ml round-bottomed flask were added Pd(OAc)2 (2.3 mg, 0.01 mmol), Gorlos-Phos-HBF4 (19.0 mg, 0.04 mmol), KOAc (294.4 mg, 3.0 mmol), bis(pinacolato)diboron (305.9, 1.2 mmol), ethyl 3-chlorophthaloylglycinate (266.5, 1.0 mmol) and dioxane (2 mL). The flask was evacuated and back filled twicewith nitrogen. The reaction mixture was heated for 2.0 hr at 90˚C. The reaction was diluted with ethyl acetate (10 mL) and filtered through a short column of silica gel (eluent: 2 × 10 mL of ethyl acetate). Evaporation of the solvent and purification by column chromatography (eluent: ethyl acetate:petroleum ether = 25:75) afforded ethyl 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolyl)phthaloylglycinate (287.5 mg, 81%) ph, 2H, OCH2), 4.41 (s, 2H, NCH2), 7.81 (d, J = 8.4 Hz, 1H, Ar-H), 8.22 (d, J = 8.4 Hz, 1H, Ar-H) and 8.39 (s, 1H, Ar-H).. 13C NMR CDCl3 14.1, 24.8, 38.9, 61.8, 122.6, 129.6, 131.0, 134.0, 140.5, 167.1, 167.3 and 167.5. HRMS (ES) calculated for (M + H) C18H22BNO6: 360.16217. Found: 360.16138.
Ethyl 3-[18F]phthaloylglycinate (18): Cyclotron-produced, no carrier added[18F]fluoride ion (50 mCi) in [18O] water (225 - 350 μL) was first adsorbed onto anion exchange resin ORTG cartridge with in the concentrator module of a NanoTek System and released with a solution of K2CO3 (1.8 mg), kryptofix (12.0 mg) in MeCN/H2O (9.5:0.5 v/v, 400 μL) into 5 mL v-vial. The solution was dried by three cycles of azeotropic evaporation with MeCN (0.45 mL) at 100˚C.
To this anhydrous 18F−-K222-K+ complex was added the boronate ester 16 (21.5 mg, 0.06 mmol), Cu(OTf)2 (21.0 mg, 0.06 mmol) and Cu(OTf)2(Py)4 (4.1 mg, 0.006 mmol) and DMF (1.0 mL). The resulting mixture was stirred at 110˚C for 20 min. The product was diluted with water (10 mL) and passed through C18 Sep-Pak to remove unreacted fluoride and the product was eluted with ethyl acetate (3 mL) to obtain 0.76 GBq of Ethyl 4-[18F]fluorophthaloylglycinate (41% E.O.S). The radio-TLC was performed on silica gel plate using ethyl acetate:hexanes (1:9) as eluent.
3. CHEMISTRY AND RESULTS
Several attempts to radiofluorinate boronate ester 1 to obtain 4-(4-[18F]fluorophenyl)piracetam (2), a potential PET brain imaging agent [ 17 , 18 ], using Tredwell’s procedure [ 15 ] with 10 mol% of copper complex Cu(OTf)2(Py)4 failed in our hands. However, previously reported 4-[18F]fluoro-1,1’-biphenyl (4) was successfully prepared from 2-([1,1’-biphenyl]-4-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (3) in 68% (reported: 74%) radiochemical yield [Scheme 1].
The boronate ester 1 has a structural motif similar to glycinate (>NCH2COOR). It is known in the literature [ 19 ] that both ethyl benzoylglycinate and ethy pthaloylglycinate readily form complexes 5 and 6 respectively with transition metals such as Fe, Co, Ni, and Cu as shown in Figure 2.
Similarly boronate ester 1 can form the glycinate complex 7 by replacing two pyridines of the pyridine copper complex Cu(OTf)2(Py)4. As the pyridine copper complex is consumed to form glycinate complex 7, there is no copper available to mediate the fluorodeboronation resulting in no product formation.

Scheme 1. Attempted radio-fluorination of piracetam analogue.
![]()
Figure 2. Copper complexes of compounds with glycinate motif.
![]()
Table 1. Radiofluorination of 11 and 16 (0.06 mmol) with 10 mol% of Cu(OTf)2(Py)4 and the effect of variable amounts of Cu(OTf)2 on radiochemical yields. a: reaction conditions using the procedure reported in the literature [ 15 ]. The yields were obtained by radio-TLC using Bioscan. The identity of products was determined by comparing with the Rf values of the standards.
In order to further test this observation, we attempted to fluorinate easily accessible benzoylglycinate and phthaloylglycinate boronate esters 11 and 16 using 10 mol% ofCu(OTf)2(Py)4 As expected, both the reactions failed. However by adding Cu(OTf)2 to the reaction mixture, we observed product formation. This prompted us to systematically study the effect of added Cu(OTf)2; the results are presented in Table 1 and Figure 3. The preparation of requisite boronate esters 11 and 16 is described in Scheme 2. The condensation of 4-chlorobenzoyl chloride, 8, with ethyl glycinate hydrochloride, 9, in the presence of triethyl amine at room temperature provided ethyl 4-chlorobenzoylglycinate, 10, in quantitative yield. 4-Chlorophthalimide, 13, was obtained in 91% yield by fusing 4-chlorophthallic acid, 12, with (NH4)2CO3 at 300˚C. N-Alkylation of phthalimide 13 with ethyl 2-bromoacetate, 14, in the presence of CsCO3 and DMF as solvent at room temperature gave ethyl 4-chlorodphthaloylglycinate, 15, in 87% yield. Palladium catalyzed borolation of aryl chlorides is assisted by various phosphine ligands such as LB-Phos (dicyclohexyl-2,4,6-trimethylphenylphosphine.HBF4), Zheda-Phos (dicyclohexyl-2-[N-methyl-N’-phenylaminophenyl]
![]()
Figure 3. Influence of excess copper tiflate on radiochemical yields.
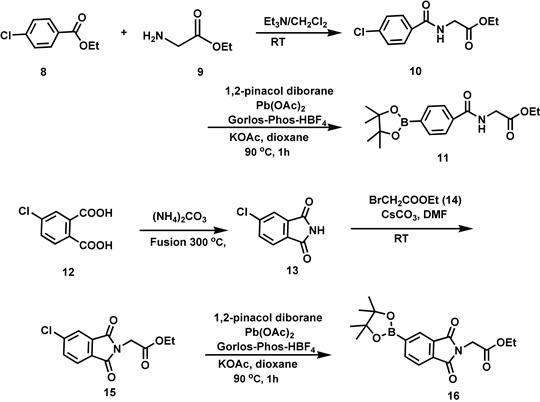
Scheme 2. Preparation of boronate precursors 11 and 16.
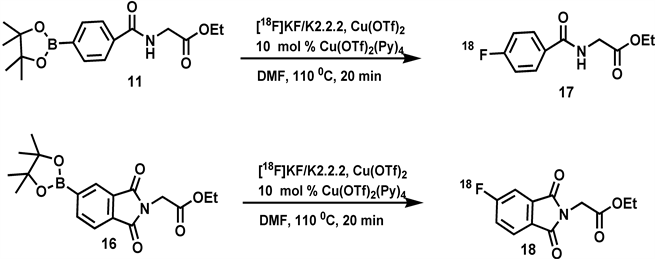
Scheme 3. Radio-fluorination of precursors 11 and 16.
phosphine.HBF4) and Gorlos-Phos (dicyclohexyl-2,6-diisopropyloxy-phenyl)phosphine.HBF4) [ 20 ]. The best yields of the boronate esters 11 (53%) and 16 (87%) were obtained by using Gorlos-Phos as ligand. No borolation took place with Zheda-Phos as it is a sterically hindered ligand. LB-Phos resulted in yields of 11 and 16 in 40% and 78% respectively. Thus aryl chlorides 10 and 15 were successfully converted to the corresponding boronate esters 11 and 16 using 3 mol% of Pd(OAc)2, 6 mol% of ligand Gorlos-Phos, 1.2 equivalents of B2Pin2 and 3 equivalents of KOAc in dioxane at 90˚C for 2 hr.
The radiofluorination of the boronate esters 11 and 16 was performed with 10 mol% of complex Cu(OTf)2(Py)4 (Scheme 3) and the effect of different mol equivalents of Cu(OTf)2 was studied. The radiolabeling of the boronate esters was carried out in the Advion NanoTek Microfludic Synthesizer. Using the drying macros of NanoTek LF 1.4 software, a complex of kryptofix 222/K2CO3/[18F]fluoride was thoroughly dried and allowed to react with the boronate esters 11 and 16 in the presence of Cu(OTf)2(Py)4 and Cu(OTf)2 in DMF at 110˚C for 20 min to obtain fluorinated compounds 17 and 18 respectively. The yields for the compounds 17 and 18 were maximized using one mole equivalent of Cu(OTf)2 in 1 mL of DMF as shown in Table 1 and Figure 3.
Following the reaction conditions from the literature [ 15 ] using 10 mol% of Cu(OTf)2(Py)4 and 0.4 ml of dimethyl formamide, no fluorination products were obtained. Fluorinated products 17 and 18 were prepared using diluted reaction mixture in 1 mL (2.5 times more diluted than literature method)) of dimethyl formamide and varying amounts of copper triflate. The highest radiochemical yields for 17 (48%) and 18 (41%) were obtained with 100 mol% of copper triflate. Radiofluorination of the boronate ester 1 resulted in relatively lower yields (20% ± 5%) of [18F]fluorophenyl piracetam 2. Further studies to improve reaction yields are underway.
4. CONCLUSIONS
Ethyl 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolyl)benzoylglycinate, 11, and ethyl 3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolyl)phthaloylglycinate, 16, were successfully radiofluorinated to obtain ethyl 4-[18F]fluorobenzoylglycinate, 17, and ethyl 3-[18F]phthaloylglycinate, 18, respectively by modifying the literature procedure. The effect of varying amounts of Cu(OTf)2 was systematically studied to improve the radiochemical yields of 17 (48%) and 18 (41%).
ACKNOWLEDGEMENTS
We wish to thank the Department of Molecular Imaging and Translational Research Program for the financial support.