Synthesis and Antifungal Activity of Some New Fluorine-Substituted 4-Thiazolidinone Bearing 1,2,4-Triazinone ()
1. Introduction
The use of heterocycles as chemical fertilizers to increase the yield of crops and to eliminate all kinds of parasites able to attack the cultivation is becoming more important because of the great problem facing the world to provide food to an increasing population [1] . Among these heterocycles, (2-thioxo-thiazolidin-4-one) and its derivatives exhibit a wide spectrum in the medicinal, pharmacological and agricultural [2] , as well as use for determination of Cu(II), Hg(II), Cl− and CN− ions in the industrial wastewater [3] . On the other hand, functionally 1,2,4-triazine derivatives have essential properties as medicinal, pharmacological and biological fields [4] [5] [6] . Also, the introduction of fluorine atom to the heterocyclic systems often enhances and improves their properties [7] [8] [9] . Based upon these observations, the present work reports synthesis of some new fluorine-substituted 4-thiazolidinone starting from 3-amino-6-(2’-aminophenyl-1,2,4-triazin-5(4H)-one (2) in view of their antifungal activity.
2. Chemistry
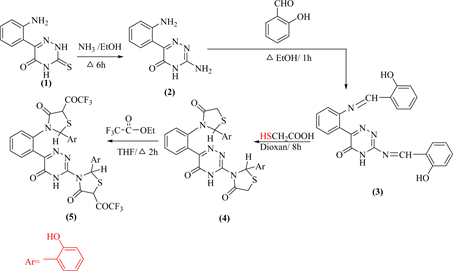
3-(Amino)-6-(2¢-aminophenyl)-1,2,4-triazin-5(4H)-one (2) prepared by aminolysis of 3-mercapto-6-(2'-aminophenyl)-1,2,4-triazin-5(4H)-one (1) [10] in reflux ethanol. Condensation of compound 2 with 2-hydroxybenzaldehyde (1:2 by moles) in reflux ethanol yielded the arylidene derivative 3 which underwent cycloaddition with thioglycolic acid in reflux dioxan afforded the 4-thiazolidi-none 4. Fluoroalkylation of compound 4 by reflux with ethyl trifluoroacetate in THF furnished 3-[(4'-oxo-2'-aryl-)-5-(trifluoroacetyl)thiazolidin-3'-yl]-6-[(2'-4"-oxo-2-aryl-5"-trifluoroacetyl)-thiazolidin-3'-yl]phenyl]-1,2.4-triazin-5(4H)-one (5) (Scheme 1). Fluoroalkylation of compound 4 takes the place of active methylene (COCH2) rather than of NH proton of 1,2,4-triazinone.
3. Results and Discussion
Former structure of compound 2 deduced from correct elemental and spectral data. The IR absorption spectrum showed ν at 3200, 3100 (NH, NH), 3080 (NH2), 1670 (C=O) and 1630 (deformation of NH2) cm−1. The 1HNMR spectrum recorded δ at 13.4, 5.5 and 3.5 ppm attributed to NH (1,2,4-triazine) and two NH2, with aromatic protons δ 7.68 - 6.9 ppm. The 13C NMR spectrum give us a good indication, that showed δ at 172.95, 147.62, 147.24 ppm for CO and two C-NH2. Also, δ at 115.48, 114.87 and 114.75 ppm for carbon of 1,2,4-triazine.
IR spectrum of compound 3 recorded a lack both NH2 functional groups, Also that 1HNMR showed a lack NH2 protons, with the presence of δ at 8.03, 8.01 ppm for two N=CH protons. On the other hand, 1H NMR spectrum of compound 4 showed a resonated CH2 protons at δ 2.68 ppm which lack’s in its of compound 5, which confirm that fluoroalkylation lack’s place at CH2 and not NH position. The IR spectrum of 5 showed mainly ν at 1250 cm−1 for C-F. The 13CNMR
Scheme 1. Synthesis of compounds 2-5.
spectrum of 5 exhibited a resonated signal at 149 ppm attribute to C-F carbons atom, with δ at 162, 158 & 152 for 3 C=O and δ at 23 ppm CH aliphatic.
4. Experimental
The melting points determined on Gallen-Kamp melting point apparatus and are uncorrected. The infrared (IR) spectra recorded on Perkin-Elmer model RXI-IR 55529. 1H and 13C NMR spectra recorded on a BurkerDPX-400 FT NMR spectrometer using tetramethylsilane as the standard internal and DMSO-d6 as solvent (chemical shift in δ, ppm). Spilling patterns designated as follows: s, singlet; d, doublet; m, multiplet. Elemental analysis performed on 2400 Perkin Elmer series 2 analyzer. Direct-MS spectra carried out using quadruple MS (Electronic ionization mod El mode with source temperature: 200˚C) at 70 eV.
4.1. 3-Amino-6-(2’-Aminophenyl)-1,2,4-Triazin-5(4H)-One (2)
To compound 1 (5 gm), liquid ammonia (39%, 50 ml), in abs. EtOH (100 ml), refluxed for 6 h, cooled. The resulted solid, filtered off and crystallized from EtOH to give 2. Yield 80%, mp: 213˚C - 216˚C. IR (ν) cm−1: 3200, 3100(NH & NH), 3080(NH2), 3020(Ar-CH), 1670 (C=O), 1630(deformation of NH2), 820 (o-substituted phenyl).1H NMR (DMSO-d6) δ ppm: 13.4, 5.5 and 3.5 (each s of 3 NH of 1,2,4-triazine), 7.68 - 6.9 (m, 4H, aromatic protons), 13C NMR (DMSO-d6) δ ppm: 172.95(C=O). 147.62(C=N), 147.24, 145(two C-NH2), 115.48, 114.87.114.75 (aromatic carbons). Analytical data, Calcd, C, 53.20; H, 4.43; N, 34.48% for C9H9N5O (203). Found: C, 52.98; N, 4.20; N, 4.20; N, 34.13%.
4.2. The Schiff Base 3
A mixture of 2 (0.01 mol) and 2-hydroxybenzaldehyde (0.02 mol) in abs. EtOH (100 ml) refluxed for 1 h, cooled. The yielded solid, filtered off and crystallized from EtOH to give 3. Yield 81%, mp: 198˚C - 202˚C. IR (ν) cm−1: 3500(OH), 3120(NH), 1610, 1580(C=N), 1660 (C=O), 850 (o-substituted phenyl). 1H NMR (DMSO-d6) δ ppm: 11.55 (s, IH, NH of 1,2,4-triazinone), 8.03, 8.01(two N=CH). 7.2 - 6.9, 6.7 - 6.5, 6.4 - 6.11(each m, 12H, aromatic protons). Analytical data, Calcd, C, 67.15; H, 4.13; N, 17.03% for C23H17N5O3. Found: C, 66.89; H, 4.01; N, 16.75%.
4.3. 3-(4-Oxo-Thiazolidin-3’-yl)-6-(2’-(4-Oxo-Thiazolidin-3’-yl) Phenyl-1,2,4-Triazin-5(4H)-One (4)
A mixture of 3(0.01 mol) and thioglycolic acid (20 ml) in dioxan (100 ml) refluxed for 8h cooled then poured onto ice. The produced solid, filtered off and crystallized from dioxane to give 4. Yield 70%, m.p: 187˚C - 190˚C. IR (ν) cm−1: 3500 - 3400 (b, OH, OH), 3120 (NH), 3030 (aromatic CH), 2980 (aliphatic CH2), 1680 - 1660 (C=O), 1380(cyclic NCSC), 1440 (deformation CH2), 900, 820 (o-substituted phenyl). Analytical data, Calcd, C, 57.96; H, 3.75; H, 3.75; N, 12.52; S, 11.44% for C27H21N5S2O5(559). Found: C, 57.77; H, 3.55; N, 12.31; S, 10.98%.
4.4. 3-[(4’-Oxo-2’-(2”-Hydroxyphenyl)-5”- (Trifluoroacetylthiazolidin-3’-yl]-6-(2’-(4”-oxo-2”-(2’”-Hydroxyphenyl)-5”-(Trifluoroacetyl)-Thiazolidin-3’-yl) Phenyl-1,2,4-Triazin-5(4H)-One (5)
A mixture of 4 (0.01 mol) and ethyl trifluoroacetate (0.02 mol) in THF (100 ml) refluxed for 1h, cooled then poured on to ice. The resulted solid, filtered off and crystallized from THF to give 5. Yield 60%, mp: 244˚C - 247˚C. IR (ν) cm−1: 3500 - 3400 (b, OH, OH), 3110 (NH), 3015 (aromatic CH), 2880 (aliphatic CH), 1710, 1700, 1680 (3 C=O), 1660 - 1650 (2 C=O of thiazolidin-4'-one), 1440 (deformation CH), 1350 (cyclic NCSC), 1250 (C-F), 910, 880,820 (o. substituted phenyl). 1H NMR (DMSO-d6) δ ppm: 12.0 (s, 1H, NH of triazine), 8.8 - 8.66, 7.12 - 7.00, 6.90 - 6.70 (each m, 12H, aromatic H), 4.8 (2H, CH-S of thiazolidinone). 12C NMR(DMSO-d6) δ ppm: 162(C=O), 158(C=O), 152(C=O), 149(C-F), 142(C=N of 1,2,4-triazine), 132-112 (aromatic carbons), 98 (C-S). Analytical data, Calcd C, 49.53; H, 2.52; N, 9.32; S, 8.52% for C31H19F6N5O7S2 (751): Found C, 49.08; H, 2.32; N, 9.11; S, 8.09 %. M/S(752, M+2, 5%), 141 (100) as COCSF3N (Figure 1).
![]()
Figure 1. Mass fragmentation pattern of compound 5.
![]()
Table 1. The antifungal activity of the un/fluorinated systems (4 & 5). Control used: DMF (30% germination).
5. Antifungal Evaluation
Firstly, in vitro, the newly prepared compounds were assayed against the growth of some phytopathogenic fungi associated with what grains, i.e. Fusarium moniliforme. The assay performed by incorporating the tested compounds with nutrient agar at different concentration. The compounds dissolved in DMF and distilled water. The poisoned media were poured into sterile Petri-dishes and allowed to solidify. Each dish inoculated with a 4 mm diameter disk of inoculum removed from a 7 day old culture of the tested pathogens. Other media supplemented with DMF serving as a control. Treatment replicated 3 times, and the plates incubated at 27˚C.
Growth on the compound amended media determined by men swing colony diameter (cm) and growth inhibition calculated with reference to the control. ED50 values determined by regression analysis of the long probit transformed data [11] [12] . From the result obtained show that compounds 5 and 4 are the most effective against the tested fungi. A higher effect of these compounds is may be due to containing fluorine atoms and/or 4-thiazolidinone moiety (Table 1).
Secondly, in vivo, the compound 5 has highest protecting activity on the grains (Tritium aestivum C.V Giza lss) of wheat against the fungal infection and increases the wheat germination compared with untreated grains. The best control of the used fungi achieved by 1000 mg/ml of compound 4 (704. Germination).
6. Conclusion
The fluorine substituted 4-thiazolidinone bearing 1,2,4-triazinone synthesized and compared with non-fluorinated were the fluorinated systems 5 exhibits highest germination (more plant protection) than the non-fluorinated systems 4 (lower plant protection).